Dual role for Headcase in hemocyte progenitor fate determination in Drosophila melanogaster
- PMID: 39466810
- PMCID: PMC11515969
- DOI: 10.1371/journal.pgen.1011448
Dual role for Headcase in hemocyte progenitor fate determination in Drosophila melanogaster
Abstract
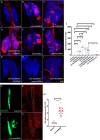
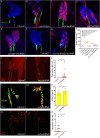
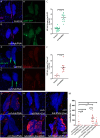
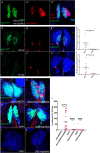
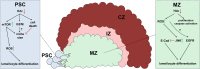
The hematopoietic organ of the Drosophila larva, the lymph gland, is a simplified representation of mammalian hematopoietic compartments, with the presence of hemocyte progenitors in the medullary zone (MZ), differentiated hemocytes in the cortical zone (CZ), and a hematopoietic niche called the posterior signaling centre (PSC) that orchestrates progenitor differentiation. Our previous work has demonstrated that the imaginal cell factor Headcase (Hdc, Heca) is required in the hematopoietic niche to control the differentiation of hemocyte progenitors. However, the downstream mechanisms of Hdc-mediated hematopoietic control remained unknown. Here we show that Hdc exerts this function by negatively regulating the insulin/mTOR signaling in the niche. When Hdc is depleted in the PSC, the overactivation of this pathway triggers reactive oxygen species (ROS) accumulation and, in turn, the differentiation of effector lamellocytes non-cell-autonomously. Although overactivation of insulin/mTOR signaling normally leads to an increase in the size of the hematopoietic niche, this effect is concealed by cell death caused by hdc loss-of-function. Moreover, we describe here that hdc silencing in progenitors causes cell-autonomous ROS elevation and JNK pathway activation, resulting in decreased MZ size and differentiation of lamellocytes. Similarly to the PSC niche, knocking down hdc in the MZ also leads to caspase activation. Notably, depleting Hdc in the progenitors triggers proliferation, an opposing effect to what is observed in the niche. These findings further our understanding of how progenitor maintenance in the larval lymph gland is controlled autonomously and non-cell-autonomously, and point towards new mechanisms potentially regulating HSC maintenance across vertebrates.
Copyright: © 2024 Kharrat et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

