Nanoplatforms for Magnetic-Photo-Heating of Thermo-Resistant Tumor Cells: Singular Synergic Therapeutic Effects at Mild Temperature
- PMID: 39466969
- PMCID: PMC11657026
- DOI: 10.1002/smll.202310522
Nanoplatforms for Magnetic-Photo-Heating of Thermo-Resistant Tumor Cells: Singular Synergic Therapeutic Effects at Mild Temperature
Abstract
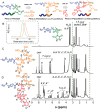
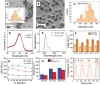
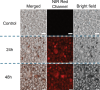
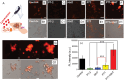
A self-assemble amphiphilic diblock copolymer that can incorporate iron oxide nanocubes (IONCs) in chain-like assemblies as heat mediators for magnetic hyperthermia (MHT) and tuneable amounts of IR780 dye as agent for photothermal therapy (PTT) is developed. MHT-heating performance of photobeads in viscous media have the same heat performances in water at magnetic field conditions of clinical use. Thanks to IR780, the photobeads are activated by infrared laser light within the first biological window (808 nm) with a significant enhancement of photo-stability of IR780 enabling the raise of the temperature at therapeutic values during multiple PTT cycles and showing unchanged optical features up to 8 days. Moreover, the photobeads fluorescent signal is preserved once internalized by glioblastoma multiforme (GBM) cells. Peculiarly, the photobeads are used as toxic agents to eradicate thermo-resistant GBM cells at mild heat, as low as 41 °C, with MHT and PTT both of clinical use. Indeed, a high U87 GBM cell mortality percentage is obtained only with dual MHT/PTT while each single treatment dose not provide the same cytotoxic effects. Only for the combined treatment, the cell death mechanism is assigned to clear sign of apoptosis as observed by structural/morphological cell studies and enhanced lysosome permeability.
Keywords: combined cancer therapy; lysosomal permeabilization; mild hyperthermia; polymeric nanostructures; thermal resistant cancer.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca‐Cancer J. Clin. 2021, 71, 209. - PubMed
-
- Miller K. D., Fidler‐Benaoudia M., Keegan T. H., Hipp H. S., Jemal A., Siegel R. L., Ca‐Cancer J. Clin. 2020, 70, 443. - PubMed
-
- Helmink B. A., Khan M. W., Hermann A., Gopalakrishnan V., Wargo J. A., Nat. Med. 2019, 25, 377. - PubMed
-
- Fan W., Yung B., Huang P., Chen X., Chem. Rev. 2017, 117, 13566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

