Pathological Microenvironment-Remodeling Nanoparticles to Alleviate Liver Fibrosis: Reversing Hepatocytes-Hepatic Stellate Cells Malignant Crosstalk
- PMID: 39467090
- PMCID: PMC11775515
- DOI: 10.1002/advs.202408898
Pathological Microenvironment-Remodeling Nanoparticles to Alleviate Liver Fibrosis: Reversing Hepatocytes-Hepatic Stellate Cells Malignant Crosstalk
Abstract
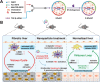
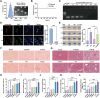
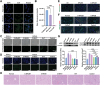
During the onset and malignant development of liver fibrosis, the pernicious interplay between damaged hepatocytes and activated hepatic stellate cells (HSCs) induce a self-perpetuating vicious cycle, deteriorating fibrosis progression and posing a grave threat to public health. The secretions released by damaged hepatocytes and activated HSCs interact through autocrine or paracrine mechanisms, involving multiple signaling pathways. This interaction creates a harsh microenvironment and weakens the therapeutic efficacy of single-cell-centric drugs. Herein, a malignant crosstalk-blocking strategy is prompted to remodel vicious cellular interplay and reverse pathological microenvironment to put an end to liver fibrosis. Collagenases modified, bardoxolone and siTGF-β co-delivered nanoparticles (C-NPs/BT) are designed to penetrate the deposited collagen barriers and further regulate the cellular interactions through upregulating anti-oxidative stress capacity and eliminating the pro-fibrogenic effects of TGF-β. The C-NPs/BT shows successful remodeling of vicious cellular crosstalk and significant disease regression in animal models. This study presents an innovative strategy to modulate cellular interactions for enhanced anti-fibrotic therapy and suggests a promising approach for treating other chronic liver diseases.
Keywords: cellular crosstalk; chemogene therapy; hepatic stellate cell; hepatocyte; liver fibrosis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hernandez‐Gea V., Friedman S. L., Annu. Rev. Pathol. Mech. Dis. 2011, 6, 425. - PubMed
-
- Trautwein C., Friedman S. L., Schuppan D., Pinzani M., J. Hepatol. 2015, 62, S15. - PubMed
-
- Devarbhavi H., Asrani S. K., Arab J. P., Nartey Y. A., Pose E., Kamath P. S., J. Hepatol. 2023, 79, 516. - PubMed
-
- Vuppalanchi R., Noureddin M., Alkhouri N., Sanyal A. J., Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373. - PubMed
MeSH terms
Substances
Grants and funding
- 82020108029/National Natural Science Foundation of China
- 82473867/National Natural Science Foundation of China
- 82073398/National Natural Science Foundation of China
- 82302367/National Natural Science Foundation of China
- 82304416/National Natural Science Foundation of China
- 2022YFE0198400/National Key R&D Program of China
- BK20232035/Natural Science Foundation of Jiangsu Basic Research Program-Project jointly funded by the province and city
- BK20231019/Natural Science Foundation of Jiangsu Province
- BK20231016/Natural Science Foundation of Jiangsu Province
- B16046/Ministry of Education of China, the State Administration of Foreign Experts Affairs of China
- 2022M720173/China Postdoctoral Science Foundation
- 2632023GR20/Fundamental Research Funds for the Central Universities
- NRF-2018R1A5A2024425/Republic of Korea
LinkOut - more resources
Full Text Sources
Medical
