A Cardiac-Targeting and Anchoring Bimetallic Cluster Nanozyme Alleviates Chemotherapy-Induced Cardiac Ferroptosis and PANoptosis
- PMID: 39467094
- PMCID: PMC11714205
- DOI: 10.1002/advs.202405597
A Cardiac-Targeting and Anchoring Bimetallic Cluster Nanozyme Alleviates Chemotherapy-Induced Cardiac Ferroptosis and PANoptosis
Abstract
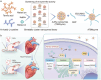
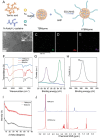
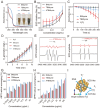
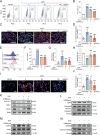
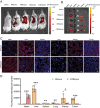
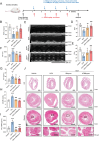
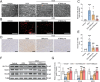
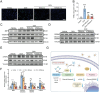
Doxorubicin (DOX), a potent antineoplastic agent, is commonly associated with cardiotoxicity, necessitating the development of strategies to reduce its adverse effects on cardiac function. Previous research has demonstrated a strong correlation between DOX-induced cardiotoxicity and the activation of oxidative stress pathways. This work introduces a novel antioxidant therapeutic approach, utilizing libraries of tannic acid and N-acetyl-L-cysteine-protected bimetallic cluster nanozymes. Through extensive screening for antioxidative enzyme-like activity, an optimal bimetallic nanozyme (AuRu) is identified that possess remarkable antioxidant characteristics, mimicking catalase-like enzymes. Theoretical calculations reveal the surface interactions of the prepared nanozymes that simulate the hydrogen peroxide decomposition process, showing that these bimetallic nanozymes readily undergo OH⁻ adsorption and O₂ desorption. To enhance cardiac targeting, the atrial natriuretic peptide is conjugated to the AuRu nanozyme. These cardiac-targeted bimetallic cluster nanozymes, with their anchoring capability, effectively reduce DOX-induced cardiomyocyte ferroptosis and PANoptosis without compromising tumor treatment efficacy. Thus, the therapeutic approach demonstrates significant reductions in chemotherapy-induced cardiac cell death and improvements in cardiac function, accompanied by exceptional in vivo biocompatibility and stability. This study presents a promising avenue for preventing chemotherapy-induced cardiotoxicity, offering potential clinical benefits for cancer patients.
Keywords: DOX‐induced cardiotoxicity; PANoptosis; antioxidant activity; bimetallic cluster nanozyme; ferroptosis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jamaledin R., Yiu C. K. Y., Zare E. N., Niu L. N., Vecchione R., Chen G., Gu Z., Tay F. R., Makvandi P., Adv. Mater. 2020, 32, 2002129. - PubMed
-
- Lena A., Wilkenshoff U., Hadzibegovic S., Porthun J., Rösnick L., Fröhlich A. K., Zeller T., Karakas M., Keller U., Ahn J., Bullinger L., Riess H., Rosen S. D., Lyon A. R., Lüscher T. F., Totzeck M., Rassaf T., Burkhoff D., Mehra M. R., Bax J. J., Butler J., Edelmann F., Haverkamp W., Anker S. D., Packer M., Coats A. J. S., von Haehling S., Landmesser U., Anker M. S., J. Am Coll. Cardiol. 2023, 81, 1569. - PubMed
-
- Ewer M. S., Ewer S. M., Nat. Rev. Cardiol. 2015, 12, 547. - PubMed
-
- Sawicki K. T., Sala V., Prever L., Hirsch E., Ardehali H., Ghigo A., Annu. Rev. Pharmacol. Toxicol. 2021, 61, 309. - PubMed
MeSH terms
Substances
Grants and funding
- 82370268/National Natural Science Foundation of China
- 222300420073/Natural Science Foundation of Henan Province
- 242300421088/Natural Science Foundation of Henan Province
- YQRC2023009/Health Science and Technology Innovation Program of Henan Province for Excellent Young Scholars
- 222301420051/Henan Provincial Science and Technology Research Project
LinkOut - more resources
Full Text Sources
