Retinal G-protein-coupled receptor deletion exacerbates AMD-like changes via the PINK1-parkin pathway under oxidative stress
- PMID: 39467145
- PMCID: PMC11580724
- DOI: 10.1096/fj.202401160RR
Retinal G-protein-coupled receptor deletion exacerbates AMD-like changes via the PINK1-parkin pathway under oxidative stress
Abstract
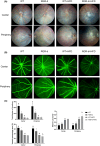
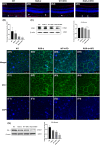
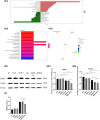
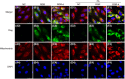
The intake of high dietary fat has been correlated with the progression of age-related macular degeneration (AMD), affecting the function of the retinal pigment epithelium through oxidative stress. A high-fat diet (HFD) can lead to lipid metabolism disorders, excessive production of circulating free fatty acids, and systemic inflammation by aggravating the degree of oxidative stress. Deletion of the retinal G-protein-coupled receptor (RGR-d) has been identified in drusen. In this study, we investigated how the RGR-d exacerbates AMD-like changes under oxidative stress, both in vivo and in vitro. Fundus atrophy became evident, at 12 months old, particularly in the RGR-d + HFD group, and fluorescence angiography revealed narrower retinal vessels and a reduced perfusion area in the peripheral retina. Although rod electroretinography revealed decreasing trends in the a- and b-wave amplitudes in the RGR-d + HFD group at 12 months, the changes were not statistically significant. Mice in the RGR-d + HFD group showed a significantly thinner and more fragile retinal morphology than those in the WT + HFD group, with disordered and discontinuous pigment distribution in the RGR-d + HFD mice. Transmission electron microscopy revealed a thickened Bruch's membrane along the choriocapillaris endothelial cell wall in the RGR-d + HFD mice, and the outer nuclear layer structure appeared disorganized, with reduced nuclear density. Kyoto Encyclopedia of Genes and Genomes pathway analysis indicated significantly lower levels of 25(OH)-vitamin D3 metabolites in the RGR-d + HFD group. Under oxidative stress, RGR-d localized to the mitochondria and reduced the levels of the PINK1-parkin pathway. RGR-d mice fed an HFD were used as a new animal model of dry AMD. Under high-fat-induced oxidative stress, RGR-d accumulated in the mitochondria, disrupting normal mitophagy and causing cellular damage, thus exacerbating AMD-like changes both in vivo and in vitro.
Keywords: AMD; RGR‐d; metabolomic; mitochondria; mitophagy.
© 2024 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Splice Variant of Retinal G-Protein-Coupled Receptor Deletion-Mediated Dysregulation of Autophagy Increases the Susceptibility to Age-Related Macular Degeneration-Like Defects.Ophthalmic Res. 2024;67(1):611-624. doi: 10.1159/000541991. Epub 2024 Oct 15. Ophthalmic Res. 2024. PMID: 39406195
-
Human RGR Gene and Associated Features of Age-Related Macular Degeneration in Models of Retina-Choriocapillaris Atrophy.Am J Pathol. 2021 Aug;191(8):1454-1473. doi: 10.1016/j.ajpath.2021.05.003. Epub 2021 May 19. Am J Pathol. 2021. PMID: 34022179 Free PMC article.
-
Proteopathy Linked to Exon-Skipping Isoform of RGR-Opsin Contributes to the Pathogenesis of Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):41. doi: 10.1167/iovs.64.13.41. Invest Ophthalmol Vis Sci. 2023. PMID: 37883094 Free PMC article.
-
Signaling Pathways in Retinal Pigment Epithelium (RPE) Cells in Response to Stress Conditions of Age-Related Macular Degeneration (AMD).Adv Exp Med Biol. 2025;1468:345-349. doi: 10.1007/978-3-031-76550-6_57. Adv Exp Med Biol. 2025. PMID: 39930220 Review.
-
Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration.Prog Retin Eye Res. 2020 Nov;79:100858. doi: 10.1016/j.preteyeres.2020.100858. Epub 2020 Apr 13. Prog Retin Eye Res. 2020. PMID: 32298788 Free PMC article. Review.
Cited by
-
Epigenetic Alterations in Age-Related Macular Degeneration: Mechanisms and Implications.Int J Mol Sci. 2025 Aug 6;26(15):7601. doi: 10.3390/ijms26157601. Int J Mol Sci. 2025. PMID: 40806731 Free PMC article. Review.
-
High-fat diet activates pyroptosis of retinal pigment epithelial cells in aged TgAPPswePS1 transgenic mice.Eur J Med Res. 2025 Jul 16;30(1):635. doi: 10.1186/s40001-025-02898-5. Eur J Med Res. 2025. PMID: 40671142 Free PMC article.
-
Retinal Autophagy for Sustaining Retinal Integrity as a Proof of Concept for Age-Related Macular Degeneration.Int J Mol Sci. 2025 Jun 16;26(12):5773. doi: 10.3390/ijms26125773. Int J Mol Sci. 2025. PMID: 40565235 Free PMC article. Review.
References
-
- Guymer RH, Campbell TG. Age‐related macular degeneration. Lancet. 2023;401(10386):1459‐1472. - PubMed
-
- Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595‐606. - PubMed
-
- Khan KN, Mahroo OA, Khan RS, et al. Differentiating drusen: drusen and drusen‐like appearances associated with ageing, age‐related macular degeneration, inherited eye disease and other pathological processes. Prog Retin Eye Res. 2016;53:70‐106. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

