Deciphering the Multifaceted Immune Landscape of Unresectable Primary Liver Cancer to Predict Immunotherapy Response
- PMID: 39467150
- PMCID: PMC11653612
- DOI: 10.1002/advs.202309631
Deciphering the Multifaceted Immune Landscape of Unresectable Primary Liver Cancer to Predict Immunotherapy Response
Abstract
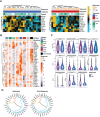
Immunotherapies employing PD-1/PD-L1 immune checkpoint inhibitors (ICIs) are vital for primary liver cancer (PLC), but response rates remain unsatisfying. Accurate differentiation of responders from non-responders to immunotherapy is imperative. Here, single-cell-scaled mass cytometry analysis on sequential peripheral blood mononuclear cells (PBMCs) from ICI-treated PLC patients is conducted, and tissue residence of immune subpopulations is assessed via multiplex immunohistochemistry. In the discovery cohort (n = 24), responders have lower baseline B cell and HLA-DR+CD8+T cell, and higher CD14+CD16- classical monocyte (CM) proportions. CMs decrease more in responders PBMCs, while HLA-DR+CD8+T cells conformably amplify after ICI-exposure. Responsive individuals display upregulated exhaustion and activation markers in peripheral immune lineages. In the expanded cohort of 77 patients, the augment of the B cells in non-responders is re-confirmed. Responders demonstrate much higher enrichment of B cells or tertiary lymphoid structures in tumor compared to non-responders. A prospective model that excelled in early discrimination of responders is developed using generalized linear model and achieves a satisfactory AUC over 0.9 in all three independent cohorts. Integratedly, the study unveils dynamic immune landscapes in PLC patients undergoing ICI-based therapy, aiding in PLC patient stratification for ICI-based treatment and fostering new response monitoring strategies.
Keywords: efficacy prediction model; immune‐checkpoint inhibition‐based therapy; peripheral immune landscapes; primary liver cancer.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zhu A. X., Abbas A. R., de Galarreta M. R., Guan Y., Lu S., Koeppen H., Zhang W., Hsu C. H., He A. R., Ryoo B. Y., Yau T., Kaseb A. O., Burgoyne A. M., Dayyani F., Spahn J., Verret W., Finn R. S., Toh H. C., Lujambio A., Wang Y., Nat. Med. 2022, 28, 1599. - PubMed
-
- Oh D.‐Y., He A. R., Qin S., Chen L.‐T., Okusaka T., Vogel A., Kim J. W., Suksombooncharoen T., Lee M. A., Kitano M., Burris Iii H. A., Bouattour M., Tanasanvimon S., Zaucha R., Avallone A., Cundom J., Rokutanda N., Xiong J., Cohen G., Valle J. W., J. Clin. Oncol. 2022, 40, 378.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
