Autoimmune Encephalitis and Paraneoplastic Neurologic Syndromes: A Nationwide Study on Epidemiology and Antibody Testing Performance
- PMID: 39467237
- PMCID: PMC11521097
- DOI: 10.1212/NXI.0000000000200318
Autoimmune Encephalitis and Paraneoplastic Neurologic Syndromes: A Nationwide Study on Epidemiology and Antibody Testing Performance
Abstract
Background and objectives: Autoimmune encephalitis (AIE) and paraneoplastic neurologic syndromes (PNSs) encompass a heterogeneous group of antibody-associated disorders. Both the number of syndromes and commercially available antibody tests have increased considerably over the past decade. High-quality population-based data on epidemiology of these disorders and real-world performance of antibody tests are needed.
Methods: In this nationwide retrospective cohort study, we identified all serum and CSF samples tested for antibodies against intracellular antigens (IAs: Hu [ANNA1], Yo [PCA1], CV2 [CRMP5], Ri [ANNA2], Ma1, Ma2 [Ta], amphiphysin, GAD65, GFAP, KLHL11, CARP VIII) or extracellular antigens (EAs: NMDAR, LGI1, Caspr2, GABA-B-R, GABA-A-R, AMPAR, DPPX, GlyR, mGluR1, VGCC, IgLON5, Tr [DNER]) between January 2016 and December 2021 in the Netherlands. Nationwide coverage was guaranteed for all antibodies except anti-GAD65 and anti-VGCC. We calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV); obtained clinical information about patients who tested positive; assigned diagnosis of AIE/PNS according to diagnostic criteria; and calculated incidence rates for IA, EA, and individual antibody-associated syndromes.
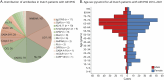
Results: In the study period, 2,877 (9.5%) of 30,246 samples, belonging to 1,228 patients, tested positive. Sensitivity and specificity were high (>95%) to very high (>99%) for most tests in both serum and CSF. PPVs for several tests were moderate to poor, especially for serum testing of IA antibodies (25%-80%). Clinical data were available for 940 (76.5%) of 1,228 patients. A total of 578 AIE/PNS diagnoses were made. The incidence rate for AIE/PNS (per million person-years) increased from 4.70 (95% CI 3.72-5.85) in 2016 to 5.76 (4.69-7.00) in 2021. Overall, the incidence rate was 5.57 (5.13-6.05), 2.96 (2.64-3.31) for the EA and 2.61 (2.31-2.94) for the IA subgroup. The 4 most common AIE/PNS types were anti-NMDAR, anti-LGI1, anti-Hu, and anti-GAD65, together comprising almost two-thirds of all diagnoses (364/578, 63.0%).
Discussion: Most commercial antibody tests perform well overall, but important pitfalls remain. Although almost all tests had high specificity, PPV was only modest in the setting of these rare diseases and mass testing. We observe trends toward increasing incidence of antibody-associated AIE/PNS.
Conflict of interest statement
J. Kerstens received a Research Mobility Fellowship from the European Joint Program on Rare Diseases (EJP-RD); M.W.J. Schreurs reports no disclosures relevant to the manuscript; J.M. de Vries has received an Autoimmune Encephalitis Alliance (AEA) Seed Grant (2021); R.F. Neuteboom reports no disclosures relevant to the manuscript; J. Brenner has been funded by Dioraphte (2001 0403); Y.S. Crijnen reports no disclosures relevant to the manuscript; R.W. van Steenhoven has been funded by ZonMw, Alzheimer Nederland, and has received an AEA Seed Grant (2024); M.A.A.M. de Bruijn has been funded by EpilepsieNL; A. van Sonderen reports no disclosures relevant to the manuscript; M.H. van Coevorden-Hameete reports no disclosures relevant to the manuscript; A.E.M. Bastiaansen has been funded by ZonMw (Memorabel program); M.R. Vermeiren reports no disclosures relevant to the manuscript; J.G.M.C. Damoiseaux reports no disclosures relevant to the manuscript; H.G. Otten reports no disclosures relevant to the manuscript; C.J.M. Frijns reports no disclosures relevant to the manuscript; B. Meek reports no disclosures relevant to the manuscript; A.C.M. Platteel reports no disclosures relevant to the manuscript; A. van de Mortel reports no disclosures relevant to the manuscript; C.C.S. Delnooz reports no disclosures relevant to the manuscript; M.A.C. Broeren reports no disclosures relevant to the manuscript; M.M. Verbeek reports no disclosures relevant to the manuscript; E.I. Hoff reports no disclosures relevant to the manuscript; S. Boukhrissi reports no disclosures relevant to the manuscript; S.C. Franken reports no disclosures relevant to the manuscript; M.M.P. Nagtzaam reports no disclosures relevant to the manuscript; M. Paunovic has been funded by an E-RARE grant; S. Veenbergen reports no disclosures relevant to the manuscript; P.A.E. Sillevis Smitt holds a patent for the detection of anti-DNER, and received research support from Euroimmun; M.J. Titulaer has filed a patent, on behalf of Erasmus MC, for methods for typing neurologic disorders and cancer, and devices for use therein, has received research funds for serving on a scientific advisory board of AmGen, for consultation at Guidepoint Global LLC, for consultation at UCB, has received an unrestricted research grant from Euroimmun AG and from CSL Behring, has received funding from EpilepsieNL. Go to
Figures
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous