LCoRL Regulates Growth and Metabolism
- PMID: 39467326
- PMCID: PMC11538781
- DOI: 10.1210/endocr/bqae146
LCoRL Regulates Growth and Metabolism
Abstract
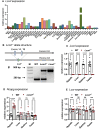
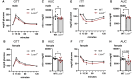
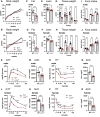
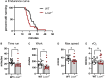
Genome-wide association studies (GWAS) in humans and livestock have identified genes associated with metabolic traits. However, the causality of many of these genes on metabolic homeostasis is largely unclear due to a lack of detailed functional analyses. Here we report ligand-dependent corepressor-like (LCoRL) as a metabolic regulator for body weight and glucose homeostasis. Although GWAS data show that LCoRL is strongly associated with body size, glucose homeostasis, and other metabolic traits in humans and livestock, functional investigations had not been performed. We generated Lcorl knockout mice (Lcorl-/-) and characterized the metabolic traits. We found that Lcorl-/- pups are born smaller than the wild-type (WT) littermates before reaching normal weight by 7 to 9 weeks of age. While aging, Lcorl-/- mice remain lean compared to WT mice, which is associated with a decrease in daily food intake. Glucose tolerance and insulin sensitivity are improved in Lcorl-/- mice. Mechanistically, this stunted growth is linked to a reduction of circulating levels of IGF-1. The expression of the genes downstream of GH signaling and the genes involved in glucose and lipid metabolism are altered in the liver of Lcorl-/- mice. Furthermore, Lcorl-/- mice are protected against a high-fat diet challenge and show reduced exercise capacity in an exercise stress test. Collectively, our results are congruent with many of the metabolic parameters linked to the Lcorl locus as reported in GWAS in humans and livestock.
Keywords: Lcorl; adiposity; body weight; exercise; glucose homeostasis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com. See the journal About page for additional terms.
Figures




References
-
- Conway E, Jerman E, Healy E, et al. A family of vertebrate-specific polycombs encoded by the LCOR/LCORL genes balance PRC2 subtype activities. Mol Cell. 2018;70(3):408‐421.e8. - PubMed
-
- Fernandes I, Bastien Y, Wai T, et al. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell. 2003;11(1):139‐150. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

