Targeting neutrophil serine proteases in bronchiectasis
- PMID: 39467608
- PMCID: PMC11694565
- DOI: 10.1183/13993003.01050-2024
Targeting neutrophil serine proteases in bronchiectasis
Abstract
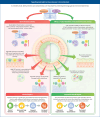
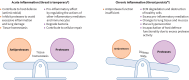
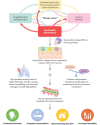
Persistent neutrophilic inflammation is a central feature in both the pathogenesis and progression of bronchiectasis. Neutrophils release neutrophil serine proteases (NSPs), such as neutrophil elastase (NE), cathepsin G and proteinase 3. When chronically high levels of free NSP activity exceed those of protective antiproteases, structural lung destruction, mucosal-related defects, further susceptibility to infection and worsening of clinical outcomes can occur. Despite the defined role of prolonged, high levels of NSPs in bronchiectasis, no drug that controls neutrophilic inflammation is licensed for the treatment of bronchiectasis. Previous methods of suppressing neutrophilic inflammation (such as direct inhibition of NE) have not been successful; however, an emerging therapy designed to address neutrophil-mediated pathology, inhibition of the cysteine protease cathepsin C (CatC, also known as dipeptidyl peptidase 1), is a promising approach to ameliorate neutrophilic inflammation, since this may reduce the activity of all NSPs implicated in bronchiectasis pathogenesis, and not just NE. Current data suggest that CatC inhibition may effectively restore the protease-antiprotease balance in bronchiectasis and improve disease outcomes as a result. Clinical trials for CatC inhibitors in bronchiectasis have reported positive phase III results. In this narrative review, we discuss the role of high NSP activity in bronchiectasis, and how this feature drives the associated morbidity and mortality seen in bronchiectasis. This review discusses therapeutic approaches aimed at treating neutrophilic inflammation in the bronchiectasis lung, summarising clinical trial outcomes and highlighting the need for more treatment strategies that effectively address chronic neutrophilic inflammation in bronchiectasis.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: J.D. Chalmers reports support for the present manuscript from Boehringer Ingelheim, grants or contracts from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed, Trudell and Novartis, consulting fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Grifols, Insmed, Janssen, Novartis, Pfizer, Trudell and Zambon, and is the current Chief Editor of the European Respiratory Journal. M.A. Mall reports support for the present manuscript from Boehringer Ingelheim, grants from Boehringer Ingelheim, the German Research Foundation (DFG), German Federal Ministry of Education and Research (BMBF), German Innovation Fund, and an independent medical grant from Vertex Pharmaceuticals with payments made to the institution, consultancy fees from AbbVie, Antabio, Arrowhead, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Prieris, Recode, Santhera, Splisense and Vertex Pharmaceuticals, personal fees for advisory board participation or consulting from AbbVie, Antabio, Arrowhead Pharmaceuticals, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Pari, Splisense and Vertex Pharmaceuticals, lecture honoraria from Vertex Pharmaceuticals, and travel support from Boehringer Ingelheim and Vertex Pharmaceuticals; M.A. Mall also reports that he is inventor on an issued patent filed by the University of North Carolina at Chapel Hill, describing the Scnn1b-transgenic mouse, and holds a leadership position as Fellow of the ERS (FERS). S.H. Chotirmall reports support for the present manuscript from Boehringer Ingelheim, grants from Singapore Ministry of Health's National Medical Research Council (Clinician Scientist Individual Research Grant (MOH-001356), Clinician Scientist Award (MOH-000710) and Open Fund Individual Research Grant (MOH-000955), and Singapore Ministry of Education under its AcRF Tier 1 Grant (RT1/22), serves on advisory boards for CSL Behring, Pneumagen Ltd and Boehringer Ingelheim, has received lecture fees from AstraZeneca and Chiesi Farmaceutici, and has served on data and safety monitoring boards for Inovio Pharmaceuticals Ltd and Imam Abdulrahman Bin Faisal University. A.E. O'Donnell reports support for the present study from Boehringer Ingelheim and Nucleus Global, grants from Boehringer Ingelheim, Insmed, Zambon, Paratek, AN2, Armata, Renovian and Spero, payment or honoraria for lectures, presentations, manuscript writing or educational events from New York University School of Medicine, Academic CME, Vinidico and Peer View Institute, and a leadership role with the US Bronchiectasis Research Registry. P.A. Flume reports support for the present study from Boehringer Ingelheim, grants or contracts from Boehringer Ingelheim, Insmed and Synchrony, consultancy fees from Insmed, and serves on advisory boards and is a site principal investigator for Insmed and Boehringer Ingelheim. N. Hasegawa reports support for the present manuscript from Boehringer Ingelheim, grants from Insmed, consulting fees from Boehringer Ingelheim and Insmed, royalties or licences from Boehringer Ingelheim, patents planned, issued or pending for Boehringer Ingelheim, and payment or honoraria for lectures from Insmed. F.C. Ringshausen reports support for the present manuscript from Boehringer Ingelheim, grants from the German Center for Lung Research (DZL), German Center for Infection Research (DZIF), IMI (EU/EFPIA) and iABC Consortium (including Alaxia, Basilea, Novartis and Polyphor), Mukoviszidose Institute, Novartis and Insmed Germany, with payments made to the institution, consulting fees from Parion Sciences, Grifols, Zambon, Insmed, Helmholtz-Zentrum für Infektionsforschung and Boehringer Ingelheim, payment or honoraria for lectures, presentations, manuscript writing or educational events from I!DE Werbeagentur GmbH, Interkongress GmbH, AstraZeneca, Insmed, Grifols and Universitätsklinikum Frankfurt am Main, payment for expert testimony from the Social Court Cologne, with payments made to the institution, support for attending meetings from Mukoviszidose eV, participation on a data and safety monitoring board or advisory board with Insmed, Grifols, Shionogi and Parion, leadership roles as PI of the German Center for Lung Research and Co-PI of the ECFS-CTN, and other financial interests from AstraZeneca, Boehringer Ingelheim, Celtaxsys, Corbus, Insmed, Novartis, Parion, University of Dundee, Vertex and Zambon, for clinical trial participation with fees paid to the institution. H. Watz reports support for the present manuscript from Boehringer Ingelheim, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi, payment or honoraria for lectures, presentations or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi, support for attending meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi, and leadership roles as Chair, COPD guideline of the German Respiratory Society and Chair, disease area COPD of the German Center for Lung Research (DZL). J-F. Xu reports support for the present manuscript from Boehringer Ingelheim. M. Shteinberg reports support for the present manuscript from Boehringer Ingelheim, grants/research support from GlaxoSmithKline, Insmed, Novartis, Trudell Pharma and Tel Aviv League for Lung Diseases, consultation fees from AstraZeneca, Boehringer Ingelheim, Dexcel, GlaxoSmithKline, Kamada, Synchrony Medical, Trumed, Vertex and Zambon, payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Insmed, Kamada, Novartis, PhysioAssist, Sanofi and Teva, participation on a data and safety monitoring board for AstraZeneca, Boehringer Ingelheim and Bonus Biotherapeutics, support for attending meetings from AstraZeneca Israel, Novartis, Actelion, Kamada, Boehringer Ingelheim, GlaxoSmithKline and Rafa, leadership roles with AJRCCM, EMBARC, the Israel Pulmonology Society and the Israel Society for TB and Mycobacterial Diseases, is an editorial board member of ERJ and Chest, and taskforce member for ERS bronchiectasis guidelines, and receipt of equipment from Trudell Medical. P.J. McShane reports support for the present manuscript from Boehringer Ingelheim, consultancy fees from Boehringer Ingelheim, payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from from Insmed, participation on a data safety monitoring board or advisory board with Boehringer Ingelheim (AIRLEAF trial), Spero and Insmed (Aspen trial), and the following financial or non-financial interests: principal investigator for Insmed (Aspen trial), Boehringer Ingelheim (AIRLEAF), Paratek (oral omadacycline), AN2 Therapeutics (Epetraborole), Renovian (ARINA-1), Spero, Armata, Electromed and MannKind.
Figures


References
-
- Laennec RTH. De l’auscultation médiate, ou Traité du diagnostic des maladies des poumons et du coeur. [On mediate auscultation, or a treatise on the diagnosis of diseases of the lungs and heart.] Paris, J-A Brosson et J-S Chaudé, 1819.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
