SF3B4 Regulates Cellular Senescence and Suppresses Therapy-induced Senescence of Cancer Cells
- PMID: 39467623
- PMCID: PMC11534033
- DOI: 10.21873/cgp.20478
SF3B4 Regulates Cellular Senescence and Suppresses Therapy-induced Senescence of Cancer Cells
Abstract
Background/aim: Cellular senescence is a state in which cells permanently exit the cell cycle, preventing tumor growth, but it can also contribute to aging and chronic inflammation. Senescence induced by cancer therapies, known as therapy-induced senescence (TIS), halts cancer cell proliferation and prevents metastasis. TIS has been investigated as an important therapeutic approach that could minimize cytotoxicity effects. This study aimed to elucidate the role of splicing factor 3B subunit 4 (SF3B4) in cellular senescence and TIS in cancer cells.
Materials and methods: β-galactosidase staining was used to examine senescence induction. SF3B4 and p21 expression were determined by RT-qPCR and western blot. Cell proliferation and cell death were evaluated.
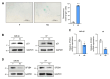
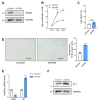
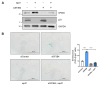
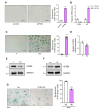
Results: SF3B4 expression decreases in replicative senescent human fibroblasts and its knockdown induces senescence via a p21-dependent pathway. In A549 non-small cell lung cancer (NSCLC) cells, SF3B4 knockdown also increased senescence markers. Notably, SF3B4 overexpression mitigated doxorubicin-induced senescence in A549 cells.
Conclusion: SF3B4 regulates senescence, and this study highlights its potential as a therapeutic target for developing better cancer treatment strategies by leveraging TIS to suppress tumor growth and enhance treatment efficacy.
Keywords: NSCLC; SF3B4; Senescence; p21; therapy-induced senescence.
Copyright © 2024, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no conflicts of interest regarding this study.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
