Norepinephrine triggers glutamatergic long-term potentiation in hypothalamic paraventricular nucleus magnocellular neuroendocrine cells through postsynaptic β1-AR/PKA signaling pathway in vitro in rats
- PMID: 39467720
- PMCID: PMC11519718
- DOI: 10.4196/kjpp.2024.28.6.569
Norepinephrine triggers glutamatergic long-term potentiation in hypothalamic paraventricular nucleus magnocellular neuroendocrine cells through postsynaptic β1-AR/PKA signaling pathway in vitro in rats
Abstract
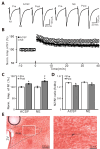
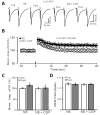
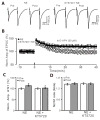
Norepinephrine (NE) modulates synaptic transmission and long-term plasticity through distinct subtype adrenergic receptor (AR)-mediated-intracellular signaling cascades. However, the role of NE modulates glutamatergic long-term potentiation (LTP) in the hypothalamic paraventricular nucleus (PVN) magnocellular neuroendocrine cells (MNCs) is unclear. We here investigate the effect of NE on high frequency stimulation (HFS)-induced glutamatergic LTP in rat hypothalamic PVN MNCs in vitro, by whole-cell patch-clamp recording, biocytin staining and pharmacological methods. Delivery of HFS induced glutamatergic LTP with a decrease in N2/N1 ratio in the PVN MNCs, which was enhanced by application of NE (100 nM). HFS-induced LTP was abolished by the blockade of N-methyl-D-aspartate receptors (NMDAR) with D-APV, but it was rescued by the application of NE. NE failed to rescue HFS-induced LTP of MNCs in the presence of a selective β1-AR antagonist, CGP 20712. However, application of β1-AR agonist, dobutamine HCl rescued HFS-induced LTP of MNCs in the absence of NMDAR activity. In the absence of NMDAR activity, NE failed to rescue HFS-induced MNC LTP when protein kinase A (PKA) was inhibited by extracellular applying KT5720 or intracellular administration of PKI. These results indicate that NE activates β1-AR and triggers HFS to induce a novel glutamatergic LTP of hypothalamic PVN NMCs via the postsynaptic PKA signaling pathway in vitro in rats.
Keywords: Adrenergic receptor; Long-term potentiation; Magnocellular neuroendocrine neuron; Paraventricular hypothalamic nucleus; Synaptic transmission.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

