The Dopamine Transporter Is a New Target for Ischemic Stroke
- PMID: 39467829
- PMCID: PMC11518691
- DOI: 10.1111/cns.70092
The Dopamine Transporter Is a New Target for Ischemic Stroke
Abstract
Aims: Dopamine transporter (DAT) can regulate DA homeostasis and has been implicated in many nervous system diseases. Whether DAT is involved in the protection against ischemic stroke is unclear.
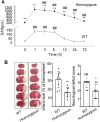
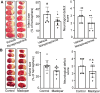
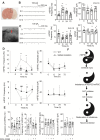
Methods: In vivo microdialysis measurements of DA were recorded in the ischemic penumbral area of mice with middle cerebral artery occlusion (MCAO). DAT coding gene, Slc6a3 mutation, and DAT overexpression animals were performed MCAO. Madopar (compound formulation of levodopa) and nomifensine (DA reuptake inhibitor) were administered in MCAO animals. Brain slices were prepared in Slc6a3 mutation or wild-type (WT) animals with MCAO to record miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs). The effects of DA and its dopamine-1 receptor (D1R) antagonists (SCH-23390) on mEPSCs, mIPSCs, and neurons protection were recorded.
Results: MCAO caused a prominent increase in DA. Slc6a3 mutation significantly attenuated the ischemic injury, whereas DAT overexpression aggravated this injury. Both nomifensine and madopar protected against brain injury. Slc6a3 mutation and DA restored the disturbance of mEPSCs and mIPSC, and protected against neuron death, which was abolished by SCH-23390.
Conclusion: DAT inhibition might be explored as a strategy for ischemic stroke prevention. DA and D1R involve in the restoration of synaptic dysfunction and neuron protection.
Keywords: D1 receptor; dopamine; dopamine transporter; ischemic stroke; miniature excitatory postsynaptic current; miniature inhibitory postsynaptic current.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
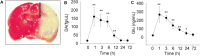

References
-
- Solinas M., Belujon P., Fernagut P. O., Jaber M., and Thiriet N., “Dopamine and Addiction: What Have We Learned From 40 Years of Research,” Journal of Neural Transmission 126 (2019): 481–516. - PubMed
-
- Gaweda G., Iyer R. P., Shaver P. R., et al., “Dopamine Receptor D3 Agonist (Pramipexole) Reduces Morphine‐Induced Cardiac Fibrosis,” Biochemical and Biophysical Research Communications 529 (2020): 1080–1085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Fund of Non- Drug Therapy Clinical Research
- 2021yygm01/Fund of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine
- A1-U21-205-902/Traditional Chinese Medicine-Clinical Evaluation Platform of Chinese Patent Medicine
- 82273984/National Natural Science Foundation of China
- 2019YFC1708504/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

