Immune Response in Traumatic Brain Injury
- PMID: 39467990
- PMCID: PMC11538248
- DOI: 10.1007/s11910-024-01382-7
Immune Response in Traumatic Brain Injury
Abstract
Purpose of review: This review aims to comprehensively examine the immune response following traumatic brain injury (TBI) and how its disruption can impact healing and recovery.
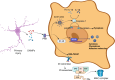
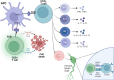
Recent findings: The immune response is now considered a key element in the pathophysiology of TBI, with consequences far beyond the acute phase after injury. A delicate equilibrium is crucial for a healthy recovery. When this equilibrium is disrupted, chronic inflammation and immune imbalance can lead to detrimental effects on survival and disability. Globally, traumatic brain injury (TBI) imposes a substantial burden in terms of both years of life lost and years lived with disability. Although its epidemiology exhibits dynamic trends over time and across regions, TBI disproportionally affects the younger populations, posing psychosocial and financial challenge for communities and families. Following the initial trauma, the primary injury is succeeded by an inflammatory response, primarily orchestrated by the innate immune system. The inflammasome plays a pivotal role during this stage, catalyzing both programmed cell death pathways and the up-regulation of inflammatory cytokines and transcription factors. These events trigger the activation and differentiation of microglia, thereby intensifying the inflammatory response to a systemic level and facilitating the migration of immune cells and edema. This inflammatory response, initially originated in the brain, is monitored by our autonomic nervous system. Through the vagus nerve and adrenergic and cholinergic receptors in various peripheral lymphoid organs and immune cells, bidirectional communication and regulation between the immune and nervous systems is established.
Keywords: Adaptive immunity; Autonomic system; Brain injury; Cholinergic pathway; Immune response; Inflammasome; Inflammation; Innate immunity; Trauma; Vagus nerve.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, Ercole A, Kunzmann K, Lanyon L, Lecky F, Lingsma H, Manley G, Nelson D, Peul W, Stocchetti N, von Steinbüchel N, Vande Vyvere T, Verheyden J, Wilson L, Maas AIR, Menon DK, CENTER-TBI Participants and Investigators. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–34. 10.1016/S1474-4422(19)30232-7. - PubMed
-
- Meyfroidt G, Bouzat P, Casaer MP, Chesnut R, Hamada SR, Helbok R, Hutchinson P, Maas AIR, Manley G, Menon DK, Newcombe VFJ, Oddo M, Robba C, Shutter L, Smith M, Steyerberg EW, Stocchetti N, Taccone FS, Wilson L, Zanier ER, Citerio G. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med. 2022;48(6):649–66. 10.1007/s00134-022-06702-4. (Erratum in: Intensive Care Med. 2022 Jul;48(7):989-991. 10.1007/s00134-022-06759-1). - PubMed
-
- Shanahan R, Avsar P, Watson C, Moore Z, Patton D, McEvoy NL, Curley G, O’Connor T. The impact of brain tissue oxygenation monitoring on the Glasgow Outcome Scale/Glasgow Outcome Scale Extended in patients with moderate to severe traumatic brain injury: A systematic review. Nurs Crit Care. 2023. 10.1111/nicc.12973. - PubMed
-
- McDonald BZ, Tarudji AW, Zhang H, Ryu S, Eskridge KM, Kievit FM. Traumatic brain injury heterogeneity affects cell death and autophagy. Exp Brain Res. 2024;242(7):1645–58. 10.1007/s00221-024-06856-1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

