SLC16A8 is a causal contributor to age-related macular degeneration risk
- PMID: 39468037
- PMCID: PMC11519927
- DOI: 10.1038/s41525-024-00442-8
SLC16A8 is a causal contributor to age-related macular degeneration risk
Abstract
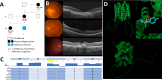
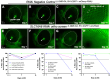
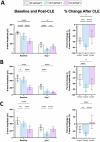
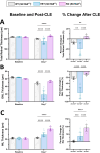
Age-related macular degeneration (AMD), a complex neurodegenerative disease, is a leading cause of visual impairment worldwide with a strong genetic component. Genetic studies have identified several loci, but few causal genes with functional characterization. Here we highlight multiple lines of evidence which show a causal role in AMD for SLC16A8, which encodes MCT3, a retinal pigment epithelium (RPE) specific lactate transporter. First, in an unbiased, genome-wide analysis of rare coding variants we show multiple SLC16A8 rare variants are associated with AMD risk, corroborating previous borderline significant reports from AMD rare variant studies. Second, we report a novel SLC16A8 mutation in a three-generation family with early onset macular degeneration. Finally, mis-expression in multiple model organisms shows functional and anatomic retinal consequences. This study highlights the important role for SLC16A8 and lactate regulation towards outer retina/RPE health and highlights a potential new therapeutic opportunity for the treatment of AMD.
© 2024. The Author(s).
Conflict of interest statement
N.N., B.H.G., A.S., T.T., G.J.K., K.C.B., Y.M., A.S., S.V.E., MIMcC, J.W.H., S.Y.C., H.J., B.L.Y. are (or were at the time of contribution) employees of Genentech/Roche with stock and stock options in Roche. N.N., H.J., B.L.Y. are listed inventors on the Genentech Inc. patent application WO2023212273A, published November 2, 2023 covering the human genetics, Drosophila melanogaster and murine model portions of the text. All other authors declare no competing interest.
Figures


References
-
- Wong, W. L. et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health2, e106–e116 (2014). - PubMed
-
- van Lookeren Campagne, M., LeCouter, J., Yaspan, B. L. & Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol.232, 151–164 (2014). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

