Using in vivo intact structure for system-wide quantitative analysis of changes in proteins
- PMID: 39468068
- PMCID: PMC11519357
- DOI: 10.1038/s41467-024-53582-x
Using in vivo intact structure for system-wide quantitative analysis of changes in proteins
Abstract
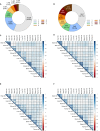
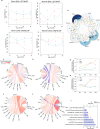
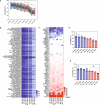
Mass spectrometry-based methods can provide a global expression profile and structural readout of proteins in complex systems. Preserving the in vivo conformation of proteins in their innate state is challenging during proteomic experiments. Here, we introduce a whole animal in vivo protein footprinting method using perfusion of reagents to add dimethyl labels to exposed lysine residues on intact proteins which provides information about protein conformation. When this approach is used to measure dynamic structural changes during Alzheimer's disease (AD) progression in a mouse model, we detect 433 proteins that undergo structural changes attributed to AD, independent of aging, across 7 tissues. We identify structural changes of co-expressed proteins and link the communities of these proteins to their biological functions. Our findings show that structural alterations of proteins precede changes in expression, thereby demonstrating the value of in vivo protein conformation measurement. Our method represents a strategy for untangling mechanisms of proteostasis dysfunction caused by protein misfolding. In vivo whole-animal footprinting should have broad applicability for discovering conformational changes in systemic diseases and for the design of therapeutic interventions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R33 CA272339/CA/NCI NIH HHS/United States
- R35 AG071734/AG/NIA NIH HHS/United States
- RF1AG061846-01/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- U01 AG088679/AG/NIA NIH HHS/United States
- 5R01AG075862/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)

