Cationized extracellular vesicles for gene delivery
- PMID: 39468145
- PMCID: PMC11519934
- DOI: 10.1038/s41598-024-75985-y
Cationized extracellular vesicles for gene delivery
Abstract
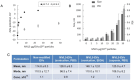
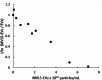
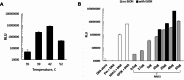
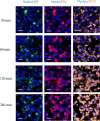
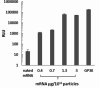
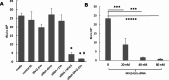
Last decade, extracellular vesicles (EVs) attracted a lot of attention as potent versatile drug delivery vehicles. We reported earlier the development of EV-based delivery systems for therapeutic proteins and small molecule chemotherapeutics. In this work, we first time engineered EVs with multivalent cationic lipids for the delivery of nucleic acids. Stable, small size cationized EVs were loaded with plasmid DNA (pDNA), or mRNA, or siRNA. Nucleic acid loaded EVs were efficiently taken up by target cells as demonstrated by confocal microscopy and delivered their cargo to the nuclei in triple negative breast cancer (TNBC) cells and macrophages. Efficient transfection was achieved by engineered cationized EVs formulations of pDNA- and mRNA in vitro. Furthermore, siRNA loaded into cationized EVs showed significant knockdown of the reporter gene in Luc-expressing cells. Overall, multivalent cationized EVs represent a promising strategy for gene delivery.
Keywords: Cancer; EVs; Gene delivery; Multivalent cationic lipid; Transfection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy.J Neuroimmune Pharmacol. 2020 Sep;15(3):487-500. doi: 10.1007/s11481-019-09884-9. Epub 2019 Nov 13. J Neuroimmune Pharmacol. 2020. PMID: 31722094
-
Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery.J Control Release. 2017 Mar 10;249:131-142. doi: 10.1016/j.jconrel.2017.01.041. Epub 2017 Feb 1. J Control Release. 2017. PMID: 28159514
-
Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery.Int J Pharm. 2020 Jan 5;573:118802. doi: 10.1016/j.ijpharm.2019.118802. Epub 2019 Nov 9. Int J Pharm. 2020. PMID: 31715354
-
Delivery of Biomolecules via Extracellular Vesicles: A Budding Therapeutic Strategy.Adv Genet. 2017;98:155-175. doi: 10.1016/bs.adgen.2017.08.002. Epub 2017 Sep 11. Adv Genet. 2017. PMID: 28942793 Review.
-
The Challenges and Possibilities of Extracellular Vesicles as Therapeutic Vehicles.Eur J Pharm Biopharm. 2019 Nov;144:50-56. doi: 10.1016/j.ejpb.2019.08.009. Epub 2019 Aug 13. Eur J Pharm Biopharm. 2019. PMID: 31419585 Review.
Cited by
-
Exosomes as Biomarkers and Therapeutic Agents in Neurodegenerative Diseases: Current Insights and Future Directions.Mol Neurobiol. 2025 Jul;62(7):9190-9215. doi: 10.1007/s12035-025-04825-5. Epub 2025 Mar 17. Mol Neurobiol. 2025. PMID: 40095345 Free PMC article. Review.
References
-
- Saraiva, C. et al. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control Release. 235, 34–47 (2016). - PubMed
-
- Movahedi, F., Hu, R. G., Becker, D. L. & Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomed. Nanotechnol. Biol. Med. 11, 1575–1584 (2015). - PubMed
-
- Dirisala, A. et al. Effective mRNA protection by poly (l-ornithine) synergizes with endosomal escape functionality of a charge‐conversion polymer toward maximizing mRNA introduction efficiency. Macromol. Rapid Commun. 43, 2100754 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

