Multiomic characterization of RNA microenvironments by oligonucleotide-mediated proximity-interactome mapping
- PMID: 39468212
- PMCID: PMC12185099
- DOI: 10.1038/s41592-024-02457-6
Multiomic characterization of RNA microenvironments by oligonucleotide-mediated proximity-interactome mapping
Abstract
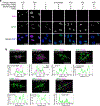
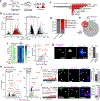
RNA molecules form complex networks of molecular interactions that are central to their function and to cellular architecture. But these interaction networks are difficult to probe in situ. Here, we introduce Oligonucleotide-mediated proximity-interactome MAPping (O-MAP), a method for elucidating the biomolecules near an RNA of interest, within its native context. O-MAP uses RNA-fluorescence in situ hybridization-like oligonucleotide probes to deliver proximity-biotinylating enzymes to a target RNA in situ, enabling nearby molecules to be enriched by streptavidin pulldown. This induces exceptionally precise biotinylation that can be easily optimized and ported to new targets or sample types. Using the noncoding RNAs 47S, 7SK and Xist as models, we develop O-MAP workflows for discovering RNA-proximal proteins, transcripts and genomic loci, yielding a multiomic characterization of these RNAs' subcellular compartments and new regulatory interactions. O-MAP requires no genetic manipulation, uses exclusively off-the-shelf parts and requires orders of magnitude fewer cells than established methods, making it accessible to most laboratories.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING FINANCIAL CONTRIBUTIONS STATEMENT
The authors declare competing financial interests. A.F.T, E.E.K, B.J.B, and D.M.S. have filed for a patent concerning the use of oligonucleotide-directed proximity-labeling to elucidate and visualize subcellular interactions
Figures



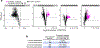










References
-
- Muller-McNicoll M & Neugebauer KM How cells get the message: dynamic assembly and function of mRNA-protein complexes. Nat Rev Genet 14, 275–287 (2013). - PubMed
-
- Hirose T, Ninomiya K, Nakagawa S & Yamazaki T A guide to membraneless organelles and their various roles in gene regulation. Nat Rev Mol Cell Biol 24, 288–304 (2023). - PubMed
METHODS-ONLY REFERENCES
-
- Eng JK, Jahan TA & Hoopmann MR Comet: an open-source MS/MS sequence database search tool. Proteomics 13, 22–24 (2013). - PubMed
MeSH terms
Substances
Grants and funding
- R37 CA241472/CA/NCI NIH HHS/United States
- R01 GM129090/GM/NIGMS NIH HHS/United States
- GM131745/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R35 GM137916/GM/NIGMS NIH HHS/United States
- S10OD021502/U.S. Department of Health & Human Services | NIH | NIH Office of the Director (OD)
- T32HG000035/U.S. Department of Health & Human Services | NIH | National Human Genome Research Institute (NHGRI)
- T32GM007750/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 HL160825/HL/NHLBI NIH HHS/United States
- 1R35GM137916/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- 1R35GM150919-01/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- 1R01HL160825-01/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 902616/American Heart Association (American Heart Association, Inc.)
- UM1 HG011586/HG/NHGRI NIH HHS/United States
- R37CA241472/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- S10 OD016240/OD/NIH HHS/United States
- R01 GM138799/GM/NIGMS NIH HHS/United States
- DEB2016186/National Science Foundation (NSF)
- T32 HG000035/HG/NHGRI NIH HHS/United States
- 1R01GM138799-01/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01GM129090/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R35 GM150919/GM/NIGMS NIH HHS/United States
- UM1HG011586/U.S. Department of Health & Human Services | NIH | National Human Genome Research Institute (NHGRI)
- T32 GM007750/GM/NIGMS NIH HHS/United States
- S10 OD021502/OD/NIH HHS/United States
- R35 GM131745/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

