Glut3 promotes cellular O-GlcNAcylation as a distinctive tumor-supportive feature in Treg cells
- PMID: 39468304
- PMCID: PMC11606946
- DOI: 10.1038/s41423-024-01229-8
Glut3 promotes cellular O-GlcNAcylation as a distinctive tumor-supportive feature in Treg cells
Abstract
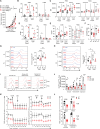
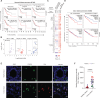
Regulatory T cells (Tregs) establish dominant immune tolerance but obstruct tumor immune surveillance, warranting context-specific mechanistic insights into the functions of tumor-infiltrating Tregs (TIL-Tregs). We show that enhanced posttranslational O-linked N-acetylglucosamine modification (O-GlcNAcylation) of cellular factors is a molecular feature that promotes a tumor-specific gene expression signature and distinguishes TIL-Tregs from their systemic counterparts. We found that altered glucose utilization through the glucose transporter Glut3 is a major facilitator of this process. Treg-specific deletion of Glut3 abrogates tumor immune tolerance, while steady-state immune homeostasis remains largely unaffected in mice. Furthermore, by employing mouse tumor models and human clinical data, we identified the NF-κB subunit c-Rel as one such factor that, through Glut3-dependent O-GlcNAcylation, functionally orchestrates gene expression in Tregs at tumor sites. Together, these results not only identify immunometabolic alterations and molecular events contributing to fundamental aspects of Treg biology, specifically at tumor sites but also reveal tumor-specific cellular properties that can aid in the development of Treg-targeted cancer immunotherapies.
Keywords: Glut3; O-GlcNAcylation; Regulatory T cells; Treg; Treg metabolism.
© 2024. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
Competing interests: Garima Sharma is an employee of ImmunoBiome Inc, South Korea. SHI is the founder and major shareholder of ImmunoBiome Inc, South Korea. SML is an employee of Lipidall Technologies Company Limited, China. The authors have no conflicting financial interests.
Figures




References
-
- Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49:1140–6. - PubMed
-
- Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–99. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

