Cost-effectiveness of oral versus injectable disease modifying therapies in relapsing multiple sclerosis: a systematic review analysis
- PMID: 39468560
- PMCID: PMC11514832
- DOI: 10.1186/s12913-024-11800-8
Cost-effectiveness of oral versus injectable disease modifying therapies in relapsing multiple sclerosis: a systematic review analysis
Erratum in
-
Correction: Cost-effectiveness of oral versus injectable disease modifying therapies in relapsing multiple sclerosis: a systematic review analysis.BMC Health Serv Res. 2025 Jan 23;25(1):129. doi: 10.1186/s12913-025-12280-0. BMC Health Serv Res. 2025. PMID: 39849552 Free PMC article. No abstract available.
Abstract
Background: Multiple sclerosis (MS) is a chronic and progressive neurological autoimmune disease that affects the central nervous system. There are two types of drugs used to treat this disease: injectable and oral drugs. The present study aimed at systematically reviewing the cost effectiveness of oral versus injectable drugs.
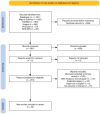
Methods: The researchers searched the PubMed, Scopus, and Web of Science databases to find relevant studies. After removing the duplicates, two authors independently assessed the records. The studies that had conducted full economic evaluations of oral versus injectable drugs in MS patients were included. The Quality of Health Economic Studies (QHES) tool was also used to assess the quality of the studies.
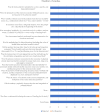
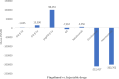
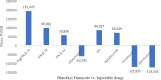
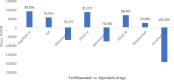
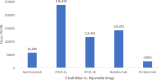
Results: Thirty studies that had conducted the economic analysis of oral versus injectable therapies in MS patients were included in this review. The QHES scores for all records were generally high (≥ 77) and they were of good quality. The lowest and highest levels of incremental net monetary benefit were respectively obtained through the comparison of Fingolimod and Alemtuzumab (-1,419,333) and the comparison of Teriflunomide and Interferon β-1a (1,792,810). The amount of INMB (incremental net monetary benefit) in the comparisons between oral and injectable drugs showed that the highest and lowest amount of INMB calculated between) Fingolimod and injectable drugs, respectively, compared to (interferon β-1a) 98,253 and (Ocrelizumab) -212,417, the highest amount in dimethyl fumarate is also against (peginterferon β-1a) 191,470 and the lowest against (alemtuzumab) -124,333, Teriflunomide against injectable drugs is the highest against (peginterferon β-1a) 89,956 and the lowest (Ocrelizumab) - 194,169, as well as Cladribine compared to injectable drugs, the highest was compared to (interferon β-1a) 236,430 and the lowest (Ocrelizumab) was 23,965.
Conclusion: A large number of health economic evaluations of disease-modifying therapies (DMTs) in MS were available at the international level, the comparison of which was difficult and sometimes contradictory. However, despite the difference in the results, Cladribine tablets were cost-effective in all studies compared with injectable drugs. In addition, the present study could be of great importance for policymakers and other beneficiaries regarding the cost-effectiveness of the aforementioned drugs.
Keywords: Cost-utility; Economic evaluation; Incremental net monetary benefit (INMB); Injectable drugs; Multiple sclerosis; Oral drugs; Quality adjusted life years (QALY).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The Impact of Price Reductions After Loss of Exclusivity in a Cost-Effectiveness Analysis: Fingolimod Versus Interferon Beta-1a for the Treatment of Relapsing-Remitting Multiple Sclerosis.J Manag Care Spec Pharm. 2019 Apr;25(4):490-498. doi: 10.18553/jmcp.2019.25.4.490. J Manag Care Spec Pharm. 2019. PMID: 30917079 Free PMC article.
-
Cost-effectiveness of oral agents in relapsing-remitting multiple sclerosis compared to interferon-based therapy in Saudi Arabia.Ann Saudi Med. 2017 Nov-Dec;37(6):433-443. doi: 10.5144/0256-4947.2017.433. Ann Saudi Med. 2017. PMID: 29229891 Free PMC article.
-
Cost-Effectiveness of Peginterferon Beta-1a and Alemtuzumab in Relapsing-Remitting Multiple Sclerosis.J Manag Care Spec Pharm. 2017 Jun;23(6):666-676. doi: 10.18553/jmcp.2017.23.6.666. J Manag Care Spec Pharm. 2017. PMID: 28530523 Free PMC article.
-
New FDA-Approved Disease-Modifying Therapies for Multiple Sclerosis.Clin Ther. 2015 Apr 1;37(4):691-715. doi: 10.1016/j.clinthera.2015.03.001. Epub 2015 Apr 4. Clin Ther. 2015. PMID: 25846320 Review.
-
Oral disease-modifying therapies for relapsing-remitting multiple sclerosis.Am J Health Syst Pharm. 2015 Jan 1;72(1):25-38. doi: 10.2146/ajhp140023. Am J Health Syst Pharm. 2015. PMID: 25511835 Review.
References
-
- Vidal-Jordana A, Montalban X. Multiple sclerosis: epidemiologic, clinical, and therapeutic aspects. Neuroimaging Clin N Am. 2017;27:195–204. - PubMed
-
- Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. 2014;48–49:134–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

