Boosting REsources And caregiver empowerment for Tracheostomy care at HomE (BREATHE) Study: study protocol for a stratified randomization trial
- PMID: 39468582
- PMCID: PMC11514889
- DOI: 10.1186/s13063-024-08522-x
Boosting REsources And caregiver empowerment for Tracheostomy care at HomE (BREATHE) Study: study protocol for a stratified randomization trial
Abstract
Background: Annually, about 4000 US children undergo a tracheostomy procedure to provide a functional, safe airway. In the hospital, qualified staff monitor and address problems, but post-discharge this responsibility shifts entirely to caregivers. The stress and constant demands of caregiving for a child with a tracheostomy with or without ventilator negatively affect caregivers. The aims of the study are to relieve the burden and stress experienced by caregivers at home, improve safety and outcomes for children post-discharge, and identify facilitators and barriers to implementation of comprehensive pediatric discharge programs.
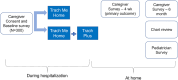
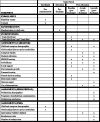
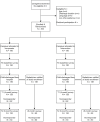
Methods: The Boosting REsources and cAregiver empowerment for Tracheostomy care at HomE (BREATHE Study) is a pragmatic two-arm, randomized trial with six sites across the US. Caregivers of a child with a tracheostomy are randomized to comparator ("Trach Me Home") or intervention ("Trach Plus"). The Comparator arm is the current gold standard focusing on caregiver education, technical skill building, and case management. The Intervention arm contains all elements of the Comparator plus educational resources, social support and communication with the outpatient pediatrician. Caregivers will complete three surveys: baseline (pre-discharge), 4-week and 6-month post-discharge. Outpatient pediatricians will complete a survey to assess self-confidence in caring for a child with tracheostomy and satisfaction with discharge communication. Interviews with clinicians and staff will identify facilitators and barriers to implementation. The study will examine whether the Intervention arm leads to lower caregiver burden, lower readmission rates and higher pediatrician satisfaction than Comparator arm.
Discussion: The BREATHE Study will advance our understanding of how hospitals can support caregivers with a child with a tracheostomy as they resume life, work, and family activities after discharge.
Trial registration: Registered on clinicaltrials.gov (NCT06283953). February 28, 2024.
Keywords: Caregiver; Discharge planning; Pediatrics; Shared decision making; Tracheostomy; Ventilator.
© 2024. The Author(s).
Conflict of interest statement
KS reports grants from Patient Centered Outcomes Research Institute (PCORI), during the conduct of the study and KS developed the Shared Decision Making Process scale (copyright Massachusetts General Hospital) that is being used as an outcome measure in the study. LS has received payment for expert witness consultation from the US Department of Justice on medical malpractice cases. LS receives research funding from PCORI to conduct studies on shared decision making between patients and clinicians.
Figures
References
-
- Muller RG, Mamidala MP, Smith SH, Smith A, Sheyn A. Incidence, Epidemiology, and Outcomes of Pediatric Tracheostomy in the United States from 2000 to 2012. Otolaryngol-Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2019;160(2):332–8. - PubMed
-
- Watters K, O’Neill M, Zhu H, Graham RJ, Hall M, Berry J. Two-year mortality, complications, and healthcare use in children with medicaid following tracheostomy. Laryngoscope. 2016;126(11):2611–7. - PubMed
-
- Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. JAMA. 2018;319(5):483–94. - PubMed
-
- Turpin DL. CONSORT and QUOROM guidelines for reporting randomized clinical trials and systematic reviews. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 2005;128(6):681–5; discussion 686. 10.1016/j.ajodo.2005.10.010. PMID: 16360902. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical