Kiss1 receptor knockout exacerbates airway hyperresponsiveness and remodeling in a mouse model of allergic asthma
- PMID: 39468619
- PMCID: PMC11520794
- DOI: 10.1186/s12931-024-03017-4
Kiss1 receptor knockout exacerbates airway hyperresponsiveness and remodeling in a mouse model of allergic asthma
Abstract
Background: In asthma, sex-steroids signaling is recognized as a critical regulator of disease pathophysiology. However, the paradoxical role of sex-steroids, especially estrogen, suggests that an upstream mechanism or even independent of estrogen plays an important role in regulating asthma pathophysiology. In this context, in our previous studies, we explored kisspeptin (Kp) and its receptor Kiss1R's signaling in regulating human airway smooth muscle cell remodeling in vitro and airway hyperresponsiveness (AHR) in vivo in a mouse (wild-type, WT) model of asthma. In this study, we evaluated the effect of endogenous Kp in regulating AHR and remodeling using Kiss1R knockout (Kiss1R-/-) mice.
Methods: C57BL/6J WT (Kiss1R+/+) and Kiss1R-/- mice, both male and female, were intranasally challenged with mixed-allergen (MA) and/or phosphate-buffered saline (PBS). We used flexiVent analysis to assess airway resistance (Rrs), elastance (Ers), and compliance (Crs). Following this, broncho-alveolar lavage (BAL) was performed for differential leukocyte count (DLC) and cytokine analysis. Histology staining was performed using hematoxylin and eosin (H&E) for morphological analysis and Masson's Trichrome (MT) for collagen deposition. Additionally, lung sections were processed for immunofluorescence (IF) of Ki-67, α-smooth muscle actin (α-SMA), and tenascin-c.
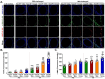
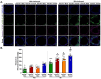
Results: Interestingly, the loss of Kiss1R exacerbated lung function and airway contractility in mice challenged with MA, with more profound effects in Kiss1R-/- female mice. MA-challenged Kiss1R-/- mice showed a significant increase in immune cell infiltration and proinflammatory cytokine levels. Importantly, the loss of Kiss1R aggravated Th2/Th17 biased cytokines in MA-challenged mice. Furthermore, histology of lung sections from Kiss1R-/- mice showed increased collagen deposition on airway walls and mucin production in airway cells compared to Kiss1R+/+ mice. In addition, immunofluorescence analysis showed loss of Kiss1R significantly aggravated airway remodeling and subsequently AHR.
Conclusions: These findings demonstrate the importance of inherent Kiss1R signaling in regulating airway inflammation, AHR, and remodeling in the pathophysiology of asthma.
Keywords: Airway inflammation; Airway smooth muscle; Asthma; FlexiVent; Lung function; α-smooth muscle actin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

