The case for microbial intervention at weaning
- PMID: 39468827
- PMCID: PMC11540084
- DOI: 10.1080/19490976.2024.2414798
The case for microbial intervention at weaning
Abstract
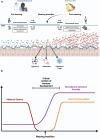
Weaning, the transition from a milk-based diet to solid food, coincides with the most significant shift in gut microbiome composition in the lifetime of most mammals. Notably, this period also marks a "window of opportunity" where key components of the immune system develop, and host-microbe interactions shape long-term immune homeostasis thereby influencing the risk of autoimmune and inflammatory diseases. This review provides a comprehensive analysis of the changes in nutrition, microbiota, and host physiology that occur during weaning. We explore how these weaning-associated processes differ across species, lifestyles, and regions of the intestine. Using prinicples of microbial ecology, we propose that the weaning transition is an optimal period for microbiome-targeted therapeutic interventions. Additionally, we suggest that replicating features of the weaning microbiome in adults could promote the successful engraftment of probiotics. Finally, we highlight key research areas that could deepen our understanding of the complex relationships between diet, commensal microbes, and the host, informing the development of more effective microbial therapies.
Keywords: Microbiome; complementary feeding; immune system development; microbial ecology; probiotics; weaning.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Silverman M, Kua L, Tanca A, Pala M, Palomba A, Tanes C, Bittinger K, Uzzau S, Benoist C, Mathis D. Protective major histocompatibility complex allele prevents type 1 diabetes by shaping the intestinal microbiota early in ontogeny. Proc Natl Acad Sci USA. 2017;114(36):9671–31. doi:10.1073/pnas.1712280114. - DOI - PMC - PubMed
-
- Galazzo G, van Best N, Bervoets L, Dapaah IO, Savelkoul PH, Hornef MW, Hutton EK, Morrison K, Holloway AC, McDonald H, et al. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology. 2020;158(6):1584–1596. doi:10.1053/j.gastro.2020.01.024. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases