Deep brain stimulation for severe dystonia associated with Wilson disease: A prospective multicenter meta-analysis of an N-of-1 trial
- PMID: 39468897
- PMCID: PMC11622510
- DOI: 10.1111/ene.16524
Deep brain stimulation for severe dystonia associated with Wilson disease: A prospective multicenter meta-analysis of an N-of-1 trial
Abstract
Background and purpose: Disabling dystonia despite optimal medical treatment is common in Wilson disease (WD). No controlled study has evaluated the effect of deep brain stimulation (DBS) on dystonia related to WD. This study was undertaken to evaluate the efficacy of DBS on dystonia related to WD.
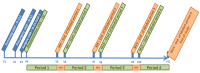
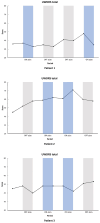
Methods: A meta-analysis of an N-of-1 prospective, randomized, double-blind, multicenter DBS study was conducted at two French WD reference centers. Main inclusion criteria were patients with WD, stabilized for at least 6 months with significant disability due to dystonia despite optimized medical treatment. The subthalamic nucleus (STN) was targeted for bradykinetic patients with tonic dystonia, and the internal globus pallidus (GPi) was chosen for patients with hyperkinetic dystonia. Each patient underwent two periods of DBS "on" and two periods of DBS "off," each lasting 4 months. The order of stimulation conditions was randomized. The primary outcome was the change in the Canadian Occupational Performance Measure Performance (COPM-P) and Satisfaction scores after each 4-month period. Secondary outcomes were changes in the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) severity and disability scores and Unified Wilson's Disease Rating Scale (UWDRS) scores.
Results: Between 12 May 2016 and 7 October 2022, three patients were included. Two patients received bilateral GPi DBS, and one received bilateral STN DBS. There was no change of COPM-P (p = 0.956), BFMDRS, and UWDRS scores. No serious adverse events were reported.
Conclusions: STN or GPi DBS are ineffective on dystonia related to WD.
Keywords: Wilson disease; deep brain stimulation; dystonia.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
C.L.: Consulting fees from Orphalan, speaker's fee from Ellivie, travel grants from Abbvie and Adelia. A.Pou.: Research (institutional) grants from Orphalan, AddMedica, and Alexion; consulting fees from Orphalan, Univar, Alexion, and Vivet Therapeutics; speaker's fees from Alexion and Orphalan. C.D.: Meeting and travel grant from Orphalan. E.R.: Honoraria for speaking from Orkyn, Aguettant, Elivie, Merz‐Pharma, Teva, and Janssen and for participating on advisory boards from Merz‐Pharma, Elivie, Teva, and BIAL; travel grants from Elivie, Merz‐Pharma, Dystonia Medical Research Foundation, International Parkinson and Movement Disorders Society, and Brazilian Society for Child Neurology. He has received research support from Merz‐Pharma, Orkyn, Elivie, Everpharma, AMADYS,
Figures
References
-
- Trocello JM, Broussolle E, Girardot‐Tinant N, et al. Wilson's disease, 100 years later…. Rev Neurol. 2013;169(12):936‐943. - PubMed
-
- Roberts EA, Schilsky ML. Current and emerging issues in Wilson's disease. N Engl J Med. 2023;389(10):922‐938. - PubMed
-
- Weiss KH, Stremmel W. Clinical considerations for an effective medical therapy in Wilson's disease. Ann N Y Acad Sci. 2014;1315(1):81‐85. - PubMed
-
- Laurencin C, Brunet AS, Dumortier J, et al. Liver transplantation in Wilson's disease with neurological impairment: evaluation in 4 patients. Eur Neurol. 2017;77(1–2):5‐15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical