Unleashing AdipoRon's Potential: A Fresh Approach to Tackle Pseudomonas aeruginosa Infections in Bronchiectasis via Sphingosine Metabolism Modulation
- PMID: 39469062
- PMCID: PMC11514707
- DOI: 10.2147/JIR.S483689
Unleashing AdipoRon's Potential: A Fresh Approach to Tackle Pseudomonas aeruginosa Infections in Bronchiectasis via Sphingosine Metabolism Modulation
Abstract
Purpose: Bronchiectasis patients are prone to Pseudomonas aeruginosa infection due to decreased level of sphingosine in airway. Adiponectin receptor agonist AdipoRon activates the intrinsic ceramidase activity of adiponectin receptor 1 (AdipoR1) and positively regulates sphingosine metabolism. This study aimed to investigate the potential therapeutic benefit of AdipoRon against Pseudomonas aeruginosa infection.
Methods: A mouse model of Pseudomonas aeruginosa lung infection and a co-culture model of human bronchial epithelial cells with Pseudomonas aeruginosa were established to explore the protective effect of AdipoRon. Liquid chromatography-mass spectrometry was used to detect the effect of AdipoRon on sphingosine level in lung of Pseudomonas aeruginosa-infected mouse models.
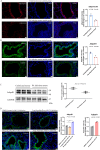
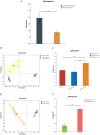
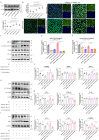
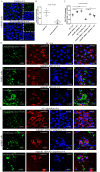
Results: The down-regulation of adiponectin and AdipoR1 in airway of bronchiectasis patients was linked to Pseudomonas aeruginosa infection. By activating AdipoR1, AdipoRon reduced Pseudomonas aeruginosa adherence on bronchial epithelial cells and protected cilia from damage in vitro. With the treatment of AdipoRon, the load of Pseudomonas aeruginosa in lung significantly decreased, and peribronchial inflammatory cell infiltration was lessened in vivo. The reduced level of sphingosine in the airway of Pseudomonas aeruginosa infected mice was replenished by AdipoRon, thus playing a protective role in the airway. Moreover, AdipoRon activated P-AMPKα/PGC1α, inhibited TLR4/P-NF-κB p65, and reduced expression of pro-apoptotic bax. However, the protective effect of AdipoRon on resisting Pseudomonas aeruginosa infection was weakened when AdipoR1 was knocked down.
Conclusion: AdipoRon protects bronchial epithelial cells and lung by enhancing their resistance to Pseudomonas aeruginosa infection. The mechanism might be modulating sphingosine metabolism and activating P-AMPKα/PGC1α while inhibiting TLR4/P-NF-κB p65.
Keywords: AdipoR1; AdipoRon; Pseudomonas aeruginosa; bronchiectasis; sphingosine metabolism.
© 2024 Xu et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials

