Efgartigimod treatment for generalized myasthenia gravis: a single-center case series of 16 patients
- PMID: 39469071
- PMCID: PMC11514137
- DOI: 10.3389/fneur.2024.1472845
Efgartigimod treatment for generalized myasthenia gravis: a single-center case series of 16 patients
Abstract
Background: Efgartigimod was approved in Japan in January 2022 for the treatment of generalized myasthenia gravis (gMG), regardless of antibody status. This case series describes a real-world experience in Japan of efgartigimod treatment for gMG patients with diverse backgrounds.
Methods: We retrospectively analyzed the medical records of 16 Japanese patients (11 females and five males, mean age 40.4 years) with gMG who received efgartigimod at the Kumamoto University Hospital between August 2022 and September 2023. The outcomes were Quantitative Myasthenia Gravis (QMG) responders (≥ 3 point reduction), IgG levels, and change in prednisolone dose, in the first cycle of efgartigimod.
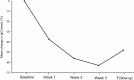
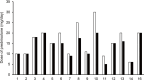
Results: Fifteen patients completed one cycle of efgartigimod. Of the 14 patients for whom QMG scores were obtained, 10 patients were QMG responders. Four of the five patients with Myasthenia Gravis Foundation of America class V were QMG responders. Improvement in QMG after efgartigimod treatment was observed in one patient with myasthenic crisis and in one refractory patient who had unsuccessful eculizumab treatment. The mean reductions from baseline in IgG levels at weeks 1, 2, 3, and follow-up were 38.3, 56.1, 63.1, and 43.9%, respectively. A decrease in prednisolone dose was observed in seven patients. The most common adverse events were headache (three patients) and diarrhea (two patients). One patient discontinued efgartigimod treatment due to a treatment-related adverse event of rash.
Conclusion: Improvements in the outcomes of patients with gMG, including patients with severe gMG, myasthenic crisis, and refractory to anti-complementary therapy, were observed after the first cycle of efgartigimod treatment. Our real-world experience in Japan suggests the future possibilities for the treatment with efgartigimod for gMG with diverse backgrounds.
Keywords: autoimmune diseases; case series; efgartigimod alfa; myasthenia gravis; neonatal Fc receptor.
Copyright © 2024 Nomura, Imamura, Imura, Mizutani and Ueda.
Conflict of interest statement
TN has received speaker honoraria from argenx. MiI received speaker honoraria from Argenx. MU has received honoraria from argenx. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Howard JF, Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. . Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1, PMID: - DOI - PubMed
-
- Mantegazza R, Wolfe GI, Muppidi S, Wiendl H, Fujita KP, O'Brien FL, et al. . Post-intervention status in patients with refractory myasthenia gravis treated with eculizumab during REGAIN and its open-label extension. Neurology. (2021) 96:e610–8. doi: 10.1212/WNL.0000000000011207, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

