A Phase 1a/1b Study of Fostroxacitabine Bralpamide (Fostrox) Monotherapy in Hepatocellular Carcinoma and Solid Tumor Liver Metastases
- PMID: 39469286
- PMCID: PMC11514655
- DOI: 10.2147/JHC.S481410
A Phase 1a/1b Study of Fostroxacitabine Bralpamide (Fostrox) Monotherapy in Hepatocellular Carcinoma and Solid Tumor Liver Metastases
Abstract
Purpose: To evaluate safety, preliminary efficacy, pharmacokinetics, and pharmacodynamics, of fostroxacitabine bralpamide (fostrox, MIV-818), a novel oral troxacitabine nucleotide prodrug designed to direct exposure to the liver, while minimizing systemic toxicity.
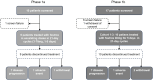
Patients and methods: Fostrox monotherapy was administered in an open-label, single-arm, first-in-human, phase 1a/1b study, in patients with hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, or solid tumor liver metastases. The first part (1a) consisted of intra/inter-patient escalating doses (3 mg to 70 mg) QD for up to 5 days, and the second part (1b), doses of 40 mg QD for 5 days, in 21-day cycles. Safety and tolerability were evaluated by the Safety Review Committee, and efficacy was assessed every 6 weeks with CT or MRI using RECIST 1.1 and mRECIST.
Results: Nineteen patients were treated with fostrox. Most common adverse events (AEs) were hematological and increased AST. Grade 3 treatment related AEs (TRAE) were seen in 53% of the patients, with transient neutropenia and thrombocytopenia as the most common. No grade 5 AE was observed. Recommended Phase 2 dose of fostrox was 40 mg QD for 5 days in 21-day cycles. Preliminary efficacy showed a clinical benefit rate in the liver of 53% and stable disease (SD) as best response in 10 patients. Liver targeting with fostrox was confirmed with higher exposure of troxacitabine and its metabolites in liver compared to plasma. Systemic exposure of fostrox was generally low with troxacitabine as main analyte. Biopsies demonstrated tumor-selective, drug-induced DNA damage.
Conclusion: The phase 1a/1b monotherapy study of fostrox, in patients with liver tumors, showed a tumor selective effect in the liver and that 40 mg QD for 5 days in 21-day cycles is safe and tolerable. Safety and preliminary efficacy in patients with advanced HCC supports clinical development of fostrox in combination with other modes of action in HCC.
Keywords: fostrox; hepatocellular carcinoma; nucleotide prodrug; pharmacodynamics; pharmacokinetics; phase 1.
© 2024 Plummer et al.
Conflict of interest statement
Professor Ruth Plummer reports personal fees from Pierre Faber, Bayer, Novartis, BMS, Ellipses, Immunocore, Genmab, Astex Therapeutics, MSD, Nerviano, AmLo, Incyte, Cybrexa, Benevolent AI, and Sanofi Aventis, outside the submitted work. Dr Debashis Sarker reports personal fees from MSD, Eisai, AstraZeneca, Ipsen, Bayer; SIRTEX, AAA, ROCHE, SERVIER, AbbVie, Incyte; non-financial support from Medivir; grants from UCB, during the conduct of the study. Dr Eric Van Cutsem reports participation to advisory boards for AbbVie, Agenus, ALX, Amgen, Arcus Biosciences, Astellas, AstraZeneca, Bayer, Beigene, Bexon Clinical, Biontech, Boehringer Ingelheim, Bristol-Myers Squibb, Canfour, Daiichi, Debiopharma, Elmedix, Eisai, Galapagos, GSK, Hookipa Biotech, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Mirati, Novartis, Nordic, Pierre Fabre, Pfizer, Roche, Seattle Genetics, Servier, Simcere, Takeda, Taiho, Terumo, outside the submitted work. Dr Beate Haugk reports royalties from Springer publication, outside the submitted work. Dr Malene Jensen is an employee and stockholder of Medivir AB. Dr Tom Morris reports personal fees from Medivir, during the conduct of the study; personal fees from AstraZeneca, Calliditas Therapeutics AB, Ascelia Pharma AB, Oxford Vacmedix Ltd, outside the submitted work. Dr Pia Baumann and Dr Karin Tunblad are employees at Medivir. Professor Fredrik Öberg is an employee and shareholder of Medivir AB. In addition, Professor Fredrik Öberg has a patent WO 2016/030335 issued to Medivir AB. Professor Thomas Evans reports grants, personal fees from Medivir, during the conduct of the study; grants, personal fees, and/or non-financial support from Astra Zeneca, Bayer, Bicycle Therapeutics, Bristol-Myers Squibb, Celgene, Eisai, MSD, Nucana, Nurix, Seagen, Pfizer, Adaptimmune, Astellas, Amgen, Avacta, Beigene, Basilea, BioNTech, Boehringer Ingelheim, Codiak, Exelixis, CytomX, Immunocore, iOnctura, Johnson & Johnson, Lilly, MiNa Therapeutics, Moderna, Novartis, Sapience, Starpharma, T3P, UCB, Verastem, Sanofi, Ascelia, Immodulon, Roche, Genmab, and Springer Nature, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures



References
-
- Detail G NCCN guidelines. 2023. Available from: https://wwwnccnorg/guidelines/guidelines-detail. Accessed October 14, 2024.
Publication types
LinkOut - more resources
Full Text Sources

