Cuprorivaite microspheres inhibit cuproptosis and oxidative stress in osteoarthritis via Wnt/β-catenin pathway
- PMID: 39469313
- PMCID: PMC11513804
- DOI: 10.1016/j.mtbio.2024.101300
Cuprorivaite microspheres inhibit cuproptosis and oxidative stress in osteoarthritis via Wnt/β-catenin pathway
Abstract
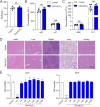
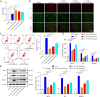
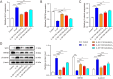
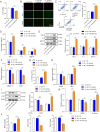
This study aims to evaluate the therapeutic potential of cuprorivaite microspheres for osteoarthritis (OA), in particular, potential molecular mechanisms were investigated. The microspheres were developed from Ca(NO3)2•4H2O, Cu(NO3)2•3H2O, and silica gel, and further therapeutic effects were tested in vitro on mouse primary chondrocytes treated with interleukin-1β (IL-1β) to mimic OA, and in vivo on OA mice induced via anterior cruciate ligament transection (ACLT) surgery. The microspheres were shown to mitigate IL-1β-induced apoptotic, inflammatory, oxidative stress and cuproptosis markers while enhancing cell viability and extracellular matrix (ECM) components in chondrocytes. Moreover, the microspheres ameliorated histopathological damage, reduced inflammatory, oxidative stress and cuproptosis markers, and enhanced ECM biomarker levels in OA mice, implicating their role in suppressing cuproptosis and oxidative stress. The aforementioned effects of the cuprorivaite microspheres were demonstrated by using SKL2001, an agonist of the Wnt/β-catenin pathway. The results suggest cuprorivaite microspheres as a promising intervention for OA and cartilage regeneration, highlighting their therapeutic effects on cellular and molecular levels.
Keywords: Cuproptosis; Cuprorivaite microspheres; Osteoarthritis; Oxidative stress; Wnt/β-catenin pathway.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Foster N.E., et al. Osteoarthritis Cartilage. 2023;31(7):876. - PubMed
-
- Falcone E., et al. Coord. Chem. Rev. 2021;433
LinkOut - more resources
Full Text Sources
