The Retention Rate and Safety of Secukinumab as a First-Line Biologic Agent in Axial Spondyloarthritis Compared to a First Tumor Necrosis Factor (TNF) Inhibitor: A Real-World, Longitudinal Study
- PMID: 39469413
- PMCID: PMC11513612
- DOI: 10.7759/cureus.70365
The Retention Rate and Safety of Secukinumab as a First-Line Biologic Agent in Axial Spondyloarthritis Compared to a First Tumor Necrosis Factor (TNF) Inhibitor: A Real-World, Longitudinal Study
Abstract
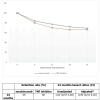
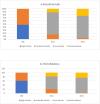
Background and objective Secukinumab (SECU) is a biologic disease-modifying antirheumatic drug (bDMARD) that has demonstrated effectiveness against axial spondyloarthritis (ax-SpA). However, in clinical practice, secukinumab is most commonly used as a second-line treatment after failure of or intolerance to tumor necrosis factor inhibitors (TNFi). In this study, we aimed to compare the two-year drug retention between secukinumab and TNFi in biologic-naïve patients with ax-SpA, to estimate the remission/low disease activity (LDA) rates in both groups and assess the safety profiles. Methods This was a longitudinal observational study involving patients with ax-SpA who were biologic-naïve and were receiving SECU or TNFi between December 2019 and December 2021. The two-year therapeutic retention rate in both groups was determined. Remission and LDA rates obtained at 24 months according to the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP) scale, as well as the safety profile, were compared between the two groups. Results Seventy-five patients were included in the study. Of them, 34.6% received SECU, while 65.3% received TNFi; 85.3% were males. The mean age was 37.8 ±9 years, the mean disease duration was 10.2 ±6.1 years, and the initial ASDAS-CRP was 3.5 ±0.8. At 24 months; the therapeutic retention rate was 70% for SECU and 66% for TNFi. The reasons for discontinuation were inefficacy (SECU: 11.5%, TNFi: 20.4%, p=0.33), side effects (SECU: 0, TNFi: 4.1%, p=0.29), and socioeconomic conditions (SECU: 15.5%, TNFi: 10.2%, p=0.51). The rate of patients achieving remission and LDA was comparable between the two groups: (remission - SECU: 23.1%, TNFi: 24.5%, p=0.92; LDA - SECU: 73.1%, TNFi: 73.5%, p=0.16). There was no statistically significant difference in the safety profile. Conclusions Our findings suggest that the effectiveness and safety of secukinumab for ax-SpA in biologic-naïve patients are comparable to those of TNFi.
Keywords: effectiveness; retention rate; secukinumab; spondyloarthritis; tnf-inhibitors.
Copyright © 2024, Zemrani et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Ethics Committee for Biomedical Research Mohammed V University- Rabat issued approval 37/24. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
One-Year Treatment Outcomes of Secukinumab Versus Tumor Necrosis Factor Inhibitors in Spondyloarthritis: Results From Five Nordic Biologic Registries Including More Than 10,000 Treatment Courses.Arthritis Care Res (Hoboken). 2022 May;74(5):748-758. doi: 10.1002/acr.24523. Epub 2022 Mar 8. Arthritis Care Res (Hoboken). 2022. PMID: 33253491
-
Retention rate and effectiveness of secukinumab vs TNF inhibitor in ankylosing spondylitis patients with prior TNF inhibitor exposure.Rheumatology (Oxford). 2021 Dec 1;60(12):5743-5752. doi: 10.1093/rheumatology/keab245. Rheumatology (Oxford). 2021. PMID: 33725088
-
Comparative Efficacy of Advanced Therapies in the Treatment of Radiographic Axial Spondyloarthritis or Ankylosing Spondylitis as Evaluated by the ASDAS Low Disease Activity Criteria.Rheumatol Ther. 2024 Aug;11(4):989-999. doi: 10.1007/s40744-024-00685-y. Epub 2024 Jun 10. Rheumatol Ther. 2024. PMID: 38858318 Free PMC article.
-
2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis.Arthritis Care Res (Hoboken). 2019 Oct;71(10):1285-1299. doi: 10.1002/acr.24025. Epub 2019 Aug 21. Arthritis Care Res (Hoboken). 2019. PMID: 31436026 Free PMC article. Review.
-
Treatment Failure in Axial Spondyloarthritis: Insights for a Standardized Definition.Adv Ther. 2022 Apr;39(4):1490-1501. doi: 10.1007/s12325-022-02064-x. Epub 2022 Feb 24. Adv Ther. 2022. PMID: 35201604 Free PMC article. Review.
References
-
- Pathogenesis of ankylosing spondylitis. Tam LS, Gu J, Yu D. Nat Rev Rheumatol. 2010;6:399–405. - PubMed
-
- Édition avril. Rabat, Morocco: Société Marocaine de Rhumatologie; 2018. Spondyloarthritis (Book in French)
-
- Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. Allez M, Karmiris K, Louis E, et al. J Crohns Colitis. 2010;4:355–366. - PubMed
-
- Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. Baeten D, Sieper J, Braun J, et al. N Engl J Med. 2015;373:2534–2548. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
