CVN424, a GPR6 inverse agonist, for Parkinson's disease and motor fluctuations: a double-blind, randomized, phase 2 trial
- PMID: 39469536
- PMCID: PMC11513664
- DOI: 10.1016/j.eclinm.2024.102882
CVN424, a GPR6 inverse agonist, for Parkinson's disease and motor fluctuations: a double-blind, randomized, phase 2 trial
Abstract
Background: CVN424 is a GPR6 inverse agonist that provides selective pharmacological control of the indirect striatopallidal pathway. We assessed the safety and efficacy of CVN424 as an adjunctive treatment to levodopa for reducing OFF-time in individuals with Parkinson's disease (PD) experiencing motor-fluctuations.
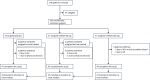
Methods: This was a randomised, double-blind, placebo-controlled study conducted at 21 sites across the United States to evaluate two doses of CVN424 (NCT04191577). Patients with PD (Hoehn and Yahr stages 2-4) who were on a stable dose of levodopa and experiencing ≥2 h of daily OFF-time were randomised (1:1:1) to receive either once-daily CVN424 (50 mg or 150 mg) or placebo for a 28-day treatment period. The primary endpoints were safety and tolerability. The key secondary endpoint was the change from baseline to Day 27 in OFF-time.
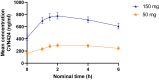
Findings: The study was conducted from December 23, 2019, to October 14, 2021. Out of 198 participants screened, 141 eligible participants were randomised to one of the three treatment groups (n = 47 per group), and 127 participants completed the 28-day treatment period. The most common treatment emergent adverse events (TEAEs) were headache (2% with CVN424 50 mg, 9% with CVN424 150 mg, and 2% with placebo) and nausea (4% with CVN424 50 mg, 6% with CVN424 150 mg and 2% with placebo). No serious treatment-related adverse events were reported. On Day 27, the mean ± standard deviation (SD) change from baseline in daily OFF-time was -1.3 ± 3.0 h in the CVN424 50 mg group, -1.6 ± 2.5 h in the CVN424 150 mg group, and -0.5 ± 2.9 h in the placebo group. The placebo-adjusted LS mean ± standard error (SE) treatment difference was significant for the CVN424 150 mg dose (1.3 ± 0.56 h, [95 CI% -2.41 to -0.19], nominal p = 0.02).
Interpretation: Treatment with CVN424 was safe and well-tolerated. Despite the short study duration and small sample size, the 150 mg CVN424 dose provided a clinically meaningful reduction in daily OFF-time. This study supports the development of CVN424 for the treatment of PD.
Funding: Cerevance.
Keywords: CVN424; GPR6; Indirect pathway; Motor fluctuations; Parkinson's disease; Striatum.
© 2024 The Authors.
Conflict of interest statement
NLB, MC, DHM, KLM, and LAD are employed by Cerevance. Martin Bexon reports consultancy to Cerevance. ALE reports honoraria and consulting fees from AbbVie, Acadia, Acorda, Adamas, Affiris, Allergan, Arbor, Biohaven, BioVie, Cerevel, Ipsen, Mitsubishi Tanabe Pharma America, NeuroDerm, Praxis, Revance, Supernus, Teva, US WorldMeds, and XW Labs. C. Warren Olanow has equity interest in Clintrex and has served as an expert witness in the paraquat litigation. JD is an adviser to Cerevance via Clintrex, and the owner of Dubow CMO Consulting, LLC. KK reports equity interest in Clintrex and Hoover Brown, patents for information management, and participation in safety monitoring boards for Roche, Lilly, and Janssen.
Figures

References
-
- Fox S.H., Katzenschlager R., Lim S.Y., et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2018;33(8):1248–1266. - PubMed
-
- Obeso J.A., Marin C., Rodriguez-Oroz C., et al. The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–S46. - PubMed
-
- Hely M.A., Morris J.G., Reid W.G., Trafficante R. Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20(2):190–199. - PubMed
-
- Warren Olanow C., Kieburtz K., Rascol O., et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord. 2013;28(8):1064–1071. - PubMed
LinkOut - more resources
Full Text Sources

