The NITRATE-OCT study-inorganic nitrate reduces in-stent restenosis in patients with stable coronary artery disease: a double-blind, randomised controlled trial
- PMID: 39469537
- PMCID: PMC11513660
- DOI: 10.1016/j.eclinm.2024.102885
The NITRATE-OCT study-inorganic nitrate reduces in-stent restenosis in patients with stable coronary artery disease: a double-blind, randomised controlled trial
Abstract
Background: Coronary angioplasty and stent insertion is a first line treatment for patients with coronary artery disease, however it is complicated in the long-term by in-stent restenosis (ISR) in a proportion of patients with an associated morbidity. Despite this, currently there are no effective treatments available for the prevention of ISR. Repeat percutaneous revascularisation carries increased risks of major adverse cardiovascular events and a higher incidence of stent failure. In this study we report the efficacy of dietary inorganic nitrate in the prevention of ISR in a prospective, double-blind, randomised controlled trial.
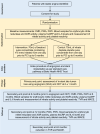
Methods: NITRATE-OCT is a double-blind, randomised, single-centre, placebo-controlled phase II trial. 300 patients who were planned to undergo percutaneous coronary intervention (PCI) and drug eluting stent (DES) implantation for stable angina were randomised on a 1:1 basis to receive a daily dose of either dietary inorganic nitrate or placebo for 6 months. Block randomisation was used and patients stratified according to diabetes status. The patients then underwent quantitative coronary angiography (QCA) at baseline and at 6 months and optical coherence tomography at 6 months to quantify ISR. The primary endpoint was the QCA quantified decrease of in-stent/in-segment diameter from the baseline measure at 6 months i.e., in-stent and in-segment late-lumen loss (LLL). The study is registered with ClinicalTrials.gov, number NCT02529189.
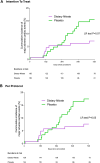
Findings: From November 1st 2015 and March 31st 2020, NITRATE-OCT enrolled 300 patients with angina, with 150 each randomised to receive 70 mL of nitrate-containing beetroot juice or placebo (nitrate-deplete) juice for 6 months. Procedural characteristics were similar between the groups. The primary endpoint was available in 208 patients: 107 and 101 in the nitrate and placebo groups, respectively. There was a statistically significant effect of inorganic nitrate on both primary endpoints: in-stent LLL decreased by 0.16 mm (95% CI:0.06-0.25; P = 0.001) with mean = 0.09 ± 0.38 mm in the inorganic nitrate group versus 0.24 ± 0.33 mm in the placebo group; (P = 0.0052); and in-segment LLL decreased by 0.24 mm (95% CI:0.12-0.36; P < 0.001) with mean = 0.02 ± 0.52 mm in the inorganic nitrate group and 0.26 ± 0.37 mm in the placebo group (P = 0.0002). Inorganic nitrate treatment was associated with a rise in the plasma nitrate concentration of ∼6.1-fold and plasma nitrite (NO2 -) of ∼2.0-fold at 6 months. These rises were associated with sustained decreases in systolic blood pressure (SBP) at 6 months compared to baseline with a change SBP of -12.06 ± 15.88 mmHg compared to the placebo group of 2.52 ± 14.60 mmHg (P < 0.0001).
Interpretation: In patients who underwent PCI for stable coronary artery disease, a once-a-day oral inorganic nitrate treatment was associated with a significant decrease in both in-stent and in-segment LLL.
Funding: This trial and KSR was funded by the National Institute for Health and Care Research (NIHR) (DRF-2014-07-008) and NIHR ACL, HW and this study were supported by The NIHR Barts Biomedical Research Centre, IC was funded by The North and East London Clinical Research Network, CL, GM were funded by The Barts Charity Cardiovascular Programme MRG00913 and MO was funded by The British Heart Foundation Project Grant PG/19/4/33995.
Keywords: Coronary; Dietary; In-stent; Nitric oxide; PCI; Restenosis.
© 2024 The Author(s).
Conflict of interest statement
Amrita Ahluwalia is a Co-Director of Heartbeet Ltd. Hector M. Garcia–Garcia has received speaker's fee from Biotronik, Boston Scientific, Medis and support for attending meetings from Biotronik, Boston Scientific. All remaining authors declare no competing interests.
Figures


References
-
- Townsend N., Kazakiewicz D., Lucy Wright F., et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19(2):133–143. - PubMed
-
- Lonborg J., Schoos M.M., Kelbaek H., et al. Impact of system delay on infarct size, myocardial salvage index, and left ventricular function in patients with ST-segment elevation myocardial infarction. Am Heart J. 2012;164(4):538–546. - PubMed
-
- Thiele H., Kappl M.J., Linke A., et al. Influence of time-to-treatment, TIMI-flow grades, and ST-segment resolution on infarct size and infarct transmurality as assessed by delayed enhancement magnetic resonance imaging. Eur Heart J. 2007;28(12):1433–1439. - PubMed
-
- Joost A., Stiermaier T., Eitel C., et al. Impact of initial culprit vessel flow on infarct size, microvascular obstruction, and myocardial salvage in acute reperfused ST-elevation myocardial infarction. Am J Cardiol. 2016;118(9):1316–1322. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

