Surviving septic patients endotyped with a functional assay demonstrate active immune responses
- PMID: 39469706
- PMCID: PMC11513262
- DOI: 10.3389/fimmu.2024.1418613
Surviving septic patients endotyped with a functional assay demonstrate active immune responses
Abstract
Introduction: Sepsis is a complex clinical syndrome characterized by a heterogenous host immune response. Historically, static protein and transcriptomic metrics have been employed to describe the underlying biology. Here, we tested the hypothesis that ex vivo functional TNF expression as well as an immunologic endotype based on both IFNγ and TNF expression could be used to model clinical outcomes in sepsis patients.
Methods: This prospective, observational study of patient samples collected from the SPIES consortium included patients at five health systems enrolled over 17 months, with 46 healthy control patients, 68 ICU patients without sepsis, and 107 ICU patients with sepsis. Whole blood was collected on day 1, 4, and 7 of ICU admission. Outcomes included in-hospital and 180-day mortality and non-favorable discharge disposition defined by skilled nursing facility, long-term acute care facility, or hospice. Whole blood ELISpot assays were conducted to quantify TNF expression [stimulated by lipopolysaccharide (LPS)] and IFNγ expression (stimulated by anti-CD3/CD28 mAb), which were then used for assignment to one of four subgroups including an 'immunocompetent', 'immunosuppressed endotype', and two 'mixed' endotypes.
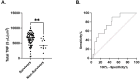
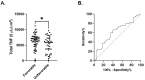
Results: Whole blood TNF spot-forming units were significantly increased in septic and CINS patients on days 4 and 7 compared to healthy subjects. In contrast, TNF expression per cell on days 1, 4, and 7 was significantly lower in both septic and critically ill non-septic (CINS) patients compared to healthy subjects. Early increases in total TNF expression were associated with favorable discharge disposition and lower in-hospital mortality. 'Immunocompetent' endotype patients on day 1 had a higher proportion of favorable to non-favorable discharges compared to the 'immunosuppressed' endotype. Similarly, 'immunocompetent' endotype patients on day 4 had a higher in-hospital survival compared to the 'immunosuppressed' endotype patients. Finally, among septic patients, decreased total TNF and IFNγ expression were associated with 180-day mortality.
Conclusions: Increased ex vivo whole blood TNF expression is associated with improved clinical outcomes. Further, the early 'immunocompetent' endotype is associated with favorable discharge and improved in-hospital and 180-day survival. The ability to functionally stratify septic patients based on blood cell function ex vivo may allow for identification of future immune modulating therapies.
Keywords: IL-6; critical illness; late mortality; prediction modeling; procalcitonin.
Copyright © 2024 Price, Becker, Barrios, Mazer, McGonagill, Bergmann, Goodman, Gould, Rao, Polcz, Kucaba, Walton, Miles, Xu, Liang, Loftus, Efron, Remy, Brakenridge, Badovinac, Griffith, Moldawer, Hotchkiss and Caldwell.
Conflict of interest statement
MM is a member of Immune Functional Diagnostics, LLC and receives no direct financial compensation. Immune Functional Diagnostics, LLC is developing predictive metrics in critical illness and this technology is evaluated in this research. KR is a member of Immune Functional Diagnostics, LLC and receives no direct financial compensation. Immune Functional Diagnostics, LLC is developing predictive metrics in critical illness and this technology is evaluated in this research. SB and the University of Florida may receive royalty income based on a technology developed by SB and others and licensed by Washington University in St. Louis to IFDx LLC. That technology is evaluated in this research. LM and the University of Florida may receive royalty income based on a technology developed by LM and others and licensed by Washington University in St. Louis to IFDx LLC. That technology is evaluated in this research. RH and Washington University in St. Louis may receive royalty income based on a technology developed by RH and others and licensed by Washington University in St. Louis to IFDx LLC. That technology is evaluated in this research. He is also supported by R35 GM-126928, awarded by the National Institute of General Medical Sciences. CC is supported by NIH grant R01GM139046. CC and the University of Cincinnati may receive royalty income based on a technology developed by CC and others and licensed by Washington University in St. Louis to IFDx LLC. That technology is evaluated in this research.
Figures


References
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. . Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644 - DOI - PubMed
-
- Dellinger R, Zimmerman J, Taylor R, Straube R, Hauser D, Criner G, et al. . Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med J. (1998) 26:15–23. doi: 10.1097/00003246-199801000-00011 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

