Ischemia-induced endogenous Nrf2/HO-1 axis activation modulates microglial polarization and restrains ischemic brain injury
- PMID: 39469715
- PMCID: PMC11513276
- DOI: 10.3389/fimmu.2024.1440592
Ischemia-induced endogenous Nrf2/HO-1 axis activation modulates microglial polarization and restrains ischemic brain injury
Abstract
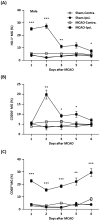
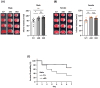
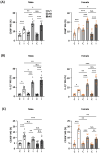
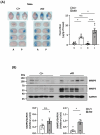
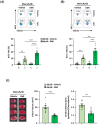
Cerebral ischemic stroke accounts for more than 80% of all stroke cases. During cerebral ischemia, reactive oxygen species produced in the ischemic brain induce oxidative stress and inflammatory responses. Nrf2 is a transcription factor responsible for regulating cellular redox balance through the induction of protective antioxidant and phase II detoxification responses. Although the induction of endogenous Nrf2/HO-1 axis activation has been observed in the ischemic brain, whether ischemia-induced endogenous Nrf2/HO-1 axis activation plays a role in modulating microglia (MG) phenotypes and restraining ischemic brain injury is not characterized and requires further exploration. To investigate that, we generated mice with Nrf2 knockdown specifically in MG to rigorously assess the role of endogenous Nrf2 activation in ischemic brain injury after stroke. Our results showed that MG-specific Nrf2 knockdown exacerbated ischemic brain injury after stroke. We found that Nrf2 knockdown altered MG phenotypes after stroke, in which increased frequency of inflammatory MG and decreased frequency of anti-inflammatory MG were detected in the ischemic brain. Moreover, we identified attenuated Nrf2/HO-1 axis activation led to increased CD68/IL-1β and suppressed CD206 expression in MG, resulting in aggravated inflammatory MG in MG-specific Nrf2 knockdown mice after stroke. Intriguingly, using type II diabetic preclinical models, we revealed that diabetic mice exhibited attenuated Nrf2/HO-1 axis activation in MG and exacerbated ischemic brain injury after stroke that phenocopy mice with MG-specific Nrf2 knockdown. Finally, the induction of exogenous Nrf2/HO-1 axis activation in MG through pharmacological approaches ameliorated ischemic brain injury in diabetic mice. In conclusion, our findings provide cellular and molecular insights demonstrating ischemia-induced endogenous Nrf2/HO-1 axis activation modulates MG phenotypes and restrains ischemic brain injury. These results further strengthen the therapeutic potential of targeting Nrf2/HO-1 axis in MG for the treatment of ischemic stroke and diabetic stroke.
Keywords: Nrf2/HO-1 axis; diabetic stroke; ischemic stroke; microglia; neuroinflammation.
Copyright © 2024 Kuo, Weng, Scofield, Paraiso, Yu and Yen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

