Cholesterol Accelerates Aggregation of α-Synuclein Simultaneously Increasing the Toxicity of Amyloid Fibrils
- PMID: 39469734
- PMCID: PMC11587506
- DOI: 10.1021/acschemneuro.4c00501
Cholesterol Accelerates Aggregation of α-Synuclein Simultaneously Increasing the Toxicity of Amyloid Fibrils
Abstract
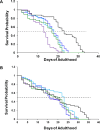
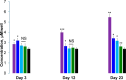
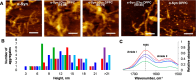
A hallmark of Parkinson disease (PD) is a progressive degeneration of neurons in the substantia nigra pars compacta, hypothalamus, and thalamus. Although the exact etiology of irreversible neuronal degeneration is unclear, a growing body of experimental evidence indicates that PD could be triggered by the abrupt aggregation of α-synuclein (α-Syn), a small membrane protein that is responsible for cell vesicle trafficking. Phospholipids uniquely alter the rate of α-Syn aggregation and, consequently, change the cytotoxicity of α-Syn oligomers and fibrils. However, the role of cholesterol in the aggregation of α-Syn remains unclear. In this study, we used Caenorhabditis elegans that overexpressed α-Syn to investigate the effect of low (15%), normal (30%), and high (60%) concentrations of cholesterol on α-Syn aggregation. We found that an increase in the concentration of cholesterol in diets substantially shortened the lifespan of C. elegans. Using biophysical methods, we also investigated the extent to which large unilamellar vesicles (LUVs) with low, normal, and high concentrations of cholesterol altered the rate of α-Syn aggregation. We found that only lipid membranes with a 60% concentration of cholesterol substantially accelerated the rate of protein aggregation. Cell assays revealed that α-Syn fibrils formed in the presence of LUVs with different concentrations of cholesterol exerted very similar levels of cytotoxicity to rat dopaminergic neurons. These results suggest that changes in the concentration of cholesterol in the plasma membrane, which in turn could be caused by nutritional preferences, could accelerate the onset and progression of PD.
Keywords: Caenorhabditis elegans; ROS; cholesterol; fibrils; α-synuclein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Chen J. Parkinson’s disease: health-related quality of life, economic cost, and implications of early treatment. Am. J. Manag. Care 2010, 16, S87–S93. - PubMed
-
- Shahmoradian S. H.; Lewis A. J.; Genoud C.; Hench J.; Moors T. E.; Navarro P. P.; Castano-Diez D.; Schweighauser G.; Graff-Meyer A.; Goldie K. N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22 (7), 1099–1109. 10.1038/s41593-019-0423-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

