Probiotics by Modulating Gut-Brain Axis Together With Brivaracetam Mitigate Seizure Progression, Behavioral Incongruities, and Prevented Neurodegeneration in Pentylenetetrazole-Kindled Mice
- PMID: 39470120
- PMCID: PMC11520030
- DOI: 10.1111/cns.70078
Probiotics by Modulating Gut-Brain Axis Together With Brivaracetam Mitigate Seizure Progression, Behavioral Incongruities, and Prevented Neurodegeneration in Pentylenetetrazole-Kindled Mice
Abstract
Background: The microbiota-gut-brain axis (MGBA) is a central nexus that integrates higher cognitive and emotional centers of the central nervous system (CNS) within the intricate functioning of the intestine. Accumulating evidence suggests that dysbiosis in the taxonomic diversity of gut flora plays a salient role in the progression of epilepsy and comorbid secondary complications.
Methods: In the current study, we investigated the impact of long-term oral bacteriotherapy (probiotics; 10 mL/kg; 109 colony-forming unit/ml) as an adjunctive treatment intervention with brivaracetam (BRV; 10 mg/kg) over 21 days on pentylenetetrazole (PTZ) induced augmented epileptic response and associated electrographical and behavioral perturbations in mice. Moreover, we also unveiled antioxidant capacity and histopathologic changes in treated versus non-treated animals.
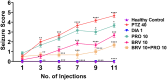
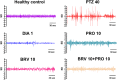
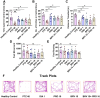
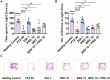
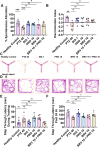
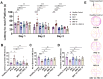
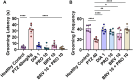
Results: Results revealed combination increases seizure threshold and prevented high ictal spiking. Additionally, it alleviated PTZ-induced neuropsychiatric disturbances such as anxiety and depressive-like phenotype along with cognitive deficits. Furthermore, dual therapy prompted physiological oxidant/antioxidant balance as evidenced by increased activity of antioxidant enzymes (SOD and catalase) and reduced levels of oxidative stressor (MDA). This therapeutic intervention with commensal species suppressed network-driven neuroinflammation and preserved normal cytoarchitecture with intact morphology in the pyramidal layers of cornu ammonis (CA1 and CA3).
Conclusion: Our study provides supporting evidence for the use of probiotics as adjunctive therapy with anti-seizure medications to modulate epileptogenic processes and related multimorbidities, particularly in individuals with drug-resistant seizures.
Keywords: EEG; Nissl's staining; PTZ kindling; epilepsy; gut–brain axis; oxidative stress; probiotics.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

