Improving adverse drug event reporting by healthcare professionals
- PMID: 39470185
- PMCID: PMC11520514
- DOI: 10.1002/14651858.CD012594.pub2
Improving adverse drug event reporting by healthcare professionals
Abstract
Background: Adverse drug events, encompassing both adverse drug reactions and medication errors, pose a significant threat to health, leading to illness and, in severe cases, death. Timely and voluntary reporting of adverse drug events by healthcare professionals plays a crucial role in mitigating the morbidity and mortality linked to unexpected reactions and improper medication usage.
Objectives: To assess the effectiveness of different interventions aimed at healthcare professionals to improve the reporting of adverse drug events.
Search methods: We searched CENTRAL, Embase, MEDLINE and several other electronic databases and trials registers, including ClinicalTrials.gov and WHO ICTRP, from inception until 14 October 2022. We also screened reference lists in the included studies and relevant systematic reviews.
Selection criteria: We included randomised trials, non-randomised controlled studies, controlled before-after studies, interrupted time series studies (ITS) and repeated measures studies, assessing the effect of any intervention aimed at healthcare professionals and designed to increase adverse drug event reporting. Eligible comparators were healthcare professionals' usual reporting practice or a different intervention or interventions designed to improve adverse drug event reporting rate. We excluded studies of interventions targeted at adverse event reporting following immunisation. Our primary outcome measures were the total number of adverse drug event reports (including both adverse drug reaction reports and medication error reports) and the number of false adverse drug event reports (encompassing both adverse drug reaction reports and medication error reports) submitted by healthcare professionals. Secondary outcomes were the number of serious, high-causality, unexpected or previously unknown, and new drug-related adverse drug event reports submitted by healthcare professionals. We used GRADE to assess the certainty of evidence.
Data collection and analysis: We followed standard methods recommended by Cochrane and the Cochrane Effective Practice and Organisation of Care (EPOC) Group. We extracted and reanalysed ITS study data and imputed treatment effect estimates (including standard errors or confidence intervals) for the randomised studies.
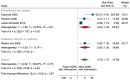
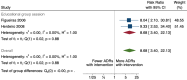
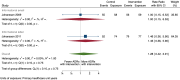
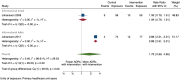
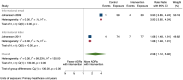
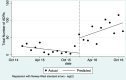
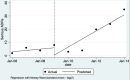
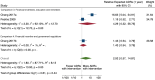
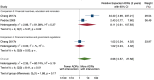
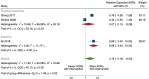
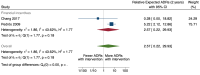
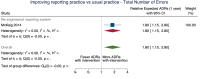
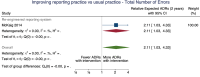
Main results: We included 15 studies (eight RCTs, six ITS, and one non-randomised cross-over study) with approximately 62,389 participants. All studies were conducted in high-income countries in large tertiary care hospitals. There was a high risk of performance bias in the controlled studies due to the nature of the interventions. None of the ITS studies had a control arm, so we could not be sure of the detected effects being independent of other changes. None of the studies reported on the number of false adverse drug event reports submitted. There is low-certainty evidence suggesting that an education session, together with reminder card and adverse drug reaction (ADR) report form, may substantially improve the rate of ADR reporting by healthcare professionals when compared to usual practice (i.e. spontaneous reporting with or without some training provided by regional pharmacosurveillance units). These educational interventions increased the number of ADR reports in total (RR 3.00, 95% CI 1.53 to 5.90; 5 studies, 21,655 participants), serious ADR reports (RR 3.30, 95% CI 1.51 to 7.21; 5 studies, 21,655 participants), high-causality ADR reports (RR 2.48, 95% CI 1.11 to 5.57; 5 studies, 21,655 participants), unexpected ADR reports (RR 4.72, 95% CI 1.75 to 12.76; 4 studies, 15,085 participants) and new drug-related ADR reports (RR 8.68, 95% CI 3.40 to 22.13; 2 studies, 7884 participants). Additionally, low-certainty evidence suggests that, compared to usual practice (i.e. spontaneous reporting), making it easier to report ADRs by using a standardised discharge form with added ADR items may slightly improve the total number of ADR reports submitted (RR 2.06, 95% CI 1.11 to 3.83; 1 study, 5967 participants). The discharge form tested was based on the 'Diagnosis Related Groups' (DRG) system for recording patient diagnoses, and the medical and surgical procedures received during their hospital stay. Due to very low-certainty evidence, we do not know if the following interventions have any effect on the total number of adverse drug event reports (including both ADR and ME reports) submitted by healthcare professionals: - sending informational letters or emails to GPs and nurses; - multifaceted interventions, including financial and non-financial incentives, fines, education and reminder cards; - implementing government regulations together with financial incentives; - including ADR report forms in quarterly bulletins and prescription pads; - providing a hyperlink to the reporting form in hospitals' electronic patient records; - improving the reporting method by re-engineering a web-based electronic error reporting system; - the presence of a clinical pharmacist in a hospital setting actively identifying adverse drug events and advocating for the identification and reporting of adverse drug events.
Authors' conclusions: Compared to usual practice (i.e. spontaneous reporting with or without some training from regional pharmacosurveillance units), low-certainty evidence suggests that the number of ADR reports submitted may substantially increase following an education session, paired with reminder card and ADR report form, and may slightly increase with the use of a standardised discharge form method that makes it easier for healthcare professionals to report ADRs. The evidence for other interventions identified in this review, such as informational letters or emails and financial incentives, is uncertain. Future studies need to assess the benefits (increase in the number of adverse drug event reports) and harms (increase in the number of false adverse drug event reports) of any intervention designed to improve healthcare professionals' reporting of adverse drug events. Interventions to increase the number of submitted adverse drug event reports that are suitable for use in low- and middle-income countries should be developed and rigorously evaluated.
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Gloria Shalviri: none known Niayesh Mohebbi: none known Fariba Mirbaha: none known Reza Majdzadeh: none known Bahareh Yazdizadeh: none known Kheirollah Gholami: none known Liesl Grobler: NHMRC, South African Medical Research Council (employment); Associate Editor of Cochrane Effective Practice and Organisation of Care (closed March 2023) but not involved in the editorial process for this review. Chris Rose: no relevant interests; Statistical Editor of Cochrane Effective Practice and Organization of Care (closed March 2023) but not involved in the editorial process for this review. Weng Yee Chin: Norwegian Institute of Public Health (independent contractor)
Figures








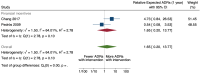
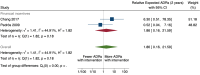
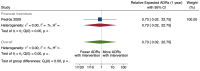




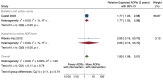

Update of
References
References to studies included in this review
Ali 2018 {published data only}
Castel 2003 {published data only}
-
- Castel JM, Figueras A, Pedros C, Laporte JR, Capella D. Stimulating adverse drug reaction reporting: effect of a drug safety bulletin and of including yellow cards in prescription pads. Drug Safety 2003;25(14):1049-55. - PubMed
Chang 2017 {published data only}
-
- Chang F, Xi Y, Zhao J, Zhang X, Lu Y. A time series analysis of the effects of financial incentives and mandatory clinical applications as interventions to improve spontaneous adverse drug reaction reporting by hospital medical staff in China. Journal of Evaluation Clinical Practice 2017;23(6):1316-21. - PubMed
Figueiras 2006 {published data only}
Hanesse 1994 {published data only}
-
- Hanesse B, Legras B, Royer RJ, Guillemin F, Briancon S. Adverse drug reactions: comparison of two report methods. Pharmacoepidemiology and Drug Safety 1994;3(4):223-9.
Herdeiro 2008 {published data only}
-
- Herdeiro MT, Polonia J, Gestal-Otero JJ, Figueiras A. Improving the reporting of adverse drug reactions: a cluster-randomized trial among pharmacists in Portugal. Drug Safety 2008;31(4):335-44. - PubMed
-
- ISRCTN45894687. An educational intervention to improve adverse drug reactions reporting: a cluster-randomised trial among Portuguese physicians and pharmacists. https://www.isrctn.com/ISRCTN45894687 (first received 7 June 2006).
Herdeiro 2012 {published data only}
-
- Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polonia J, Falcao A, Figueiras A. Workshop- and telephone-based interventions to improve adverse drug reaction reporting: a cluster-randomized trial in Portugal. Drug Safety 2012;35(8):655-65. - PubMed
Johansson 2009 {published data only}
-
- Johansson ML, Brunlof G, Edward C, Wallerstedt SM. Effects of e-mails containing ADR information and a current case report on ADR reporting rate and quality of reports. European Journal of Clinical Pharmacology 2009;65(5):511-4. - PubMed
Johansson 2011 {published data only}
Lopez‐Gonzalez 2015 {published data only}
-
- ISRCTN91140684. An educational intervention to improve adverse drug reactions reporting among Galician physicians. https://www.isrctn.com/ISRCTN91140684 (first received 7 May 2009).
-
- Lopez-Gonzalez E, Herdeiro MT, Pineiro-Lamas M, Figueiras A, Grephepi group. Effect of an educational intervention to improve adverse drug reaction reporting in physicians: a cluster randomized controlled trial. Drug Safety 2015;38(2):189-96. - PubMed
McKaig 2014 {published data only}
-
- McKaig D, Collins C, Elsaid KA. Impact of a reengineered electronic error-reporting system on medication event reporting and care process improvements at an urban medical center. Joint Commission Journal on Quality & Patient Safety 2014;40(9):398-407. - PubMed
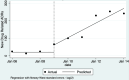
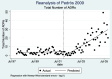
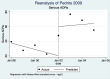
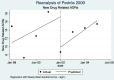
Pedrós 2009 {published data only}
-
- Pedrós C, Vallano A, Cereza G, Mendoza-Aran G, Agusti A, Aguilera C, et al. An intervention to improve spontaneous adverse drug reaction reporting by hospital physicians: a time series analysis in Spain. Drug Safety 2009;32(1):77-83. - PubMed
Ribeiro‐Vaz 2011 {published data only}
-
- Ribeiro-Vaz I, Herdeiro MT, Polonia J, Figueiras A. Strategies to increase the sensitivity of pharmacovigilance in Portugal [Estratégias para aumentar a sensibilidade da farmacovigilância em Portugal]. Revista Saude Publica 2011;45(1):129-35. - PubMed
Ribeiro‐Vaz 2012 {published data only}
-
- Ribeiro-Vaz I, Santos Cda, Costa-Pereira A, Cruz-Correia R. Promoting spontaneous adverse drug reaction reporting in hospitals using a hyperlink to the online reporting form: an ecological study in Portugal. Drug Safety 2012;33(5):387-94. - PubMed
Schlienger 1999 {published data only}
-
- Schlienger RG, Luscher TF, Schoenenberger RA, Haefeli WE. Academic detailing improves identification and reporting of adverse drug events. Pharmacy World & Science 1999;21(3):110-5. - PubMed
References to studies excluded from this review
Aldeyab 2016 {published data only}
-
- Aldeyab MA, Noble SC, Cuthbert M, Maxwell S, Dear J, Boyter A. Assessment of the impact of the Scottish public health campaign on patient reporting of adverse drug reactions. Drugs and Therapy Perspectives 2016;32(5):209-18.
Anbalagan 2019 {published data only}
-
- Anbalagan K, Shanmugasundaram P. A non-randomized interventional study to promote the knowledge, attitude and practices of community pharmacists towards pharmacovigilance. International Journal of Research in Pharmaceutical Sciences 2019;10(4):3286-92.
Åserød 2017 {published data only}
-
- Åserød H, Babic A. Designing a mobile system for safety reporting of arthroplasty adverse events. In: In: Eskola H, Väisänen O, Viik J, Hyttinen J (eds) EMBEC & NBC 2017. EMBEC NBC 2017 2017. IFMBE Proceedings. Vol. 65. Singapore: Springer, 2018:571-4. [DOI: ]
Aspinall 2002 {published data only}
-
- Aspinall MB, Whittle J, Aspinall SL, Maher Jr RL, Good CB. Improving adverse-drug-reaction reporting in ambulatory care clinics at a Veterans Affairs Hospital. American Journal of Health-System Pharmacy 2002;59(9):841-5. - PubMed
Avong 2018 {published data only}
-
- Avong YK, Jatau B, Gurumnaan R, Danat N, Okuma J, Usman I, et al. Addressing the under-reporting of adverse drug reactions in public health programs controlling HIV/AIDS, Tuberculosis and Malaria: a prospective cohort study. PLoS One 2018;13(8):e0200810. [DOI: doi: 10.1371/journal.pone.0200810. PMID: 30133453; PMCID: PMC6104922.] - PMC - PubMed
Bäckström 2002 {published data only}
-
- Bäckström M, Mjorndal T, Dahlqvist R. Spontaneous reporting of adverse drug reactions by nurses. Pharmacoepidemiology and Drug Safety 2002;11(8):647-50. - PubMed
Bäckström 2006 {published data only}
-
- Bäckström M, Mjorndal T. A small economic inducement to stimulate increased reporting of adverse drug reactions--a way of dealing with an old problem? European Journal of Clinical Pharmacology 2006;62(5):381-5. [DOI: ] - PubMed
Belhekar 2022 {published data only}
-
- Belhekar MN, Dhorajiwala SS. A prospective comparative study to evaluate the impact of educational interventions on knowledge, attitude, and practice about pharmacovigilance among nurses. National Journal of Physiology, Pharmacy and Pharmacology 2022;12(8):1164-70.
Bracchi 2005 {published data only}
-
- Bracchi RC, Houghton J, Woods FJ, Thomas S, Smail SA, Routledge PA. A distance-learning programme in pharmacovigilance linked to educational credits is associated with improved reporting of suspected adverse drug reactions via the UK yellow card scheme. British Journal of Clinical Pharmacology 2005;60(2):221-3. - PMC - PubMed
Candore 2022 {published data only}
Cano‐Sandoval 2020 {published data only}
-
- Cano-Sandoval MA, Lopez-Armas GC, Perfecto-Avalos Y, Vazquez-Alvarez AO, Brennan-Bourdon LM. Opportunities to improve the electronic reporting system for adverse drug reactions in Mexico: a comparative evaluation with the United States of America and the European Union. Pharmacoepidemiology and Drug Safety 2020;29(11):1523-6. - PubMed
Capucho 2008 {published data only}
-
- Capucho H, Reis L, Siqueira S, Pereira L. Strategies impact to increase the reporting of pharmacovigilance in a Brazilian university hospital. Drug Safety 2008;31(10):885.
Chatas 1990 {published data only}
-
- Chatas CA, Vinson B. Program for improving adverse drug reaction reporting. American Journal of Hospital Pharmacy 1990;47:155-7. - PubMed
Clarkson 2001 {published data only}
Colodny 1999 {published data only}
-
- Colodny L, Spillane J. Toward increased reporting of adverse drug reactions. Hospital Pharmacy 1999;34(10):1179-85.
Correa 2019 {published data only}
-
- Correa E, Jauregui OI, Frangella J, Peuchot V, Otero C, Luna D. Adverse drug event reporting rates after the implementation of an EHR integrated reporting system. Studies in Health Technology and Informatics 2019;21(264):1652-3. - PubMed
Costello 2007 {published data only}
-
- Costello JL, Torowicz DL, Yeh TS. Effects of a pharmacist-led pediatrics medication safety team on medication-error reporting. American Journal of Health-System Pharmacy 2007;64(13):1422-6. - PubMed
Deslandes 2022 {published data only}
Fang 2017 {published data only}
Fracchiolla 2018 {published data only}
-
- Fracchiolla NS, Artuso S, Cortelezzi A, Pelizzari AM, Tozzi P, Bonfichi M, et al. FarmaREL: an Italian pharmacovigilance project to monitor and evaluate adverse drug reactions in haematologic patients. Hematological Oncology 2018;36(1):299-306. - PubMed
Gony 2010 {published data only}
-
- Gony M, Badie K, Sommet A, Jacquot J, Baudrin D, Gauthier P, etal. Improving adverse drug reaction reporting in hospitals: results of the French Pharmacovigilance in Midi-Pyrenees region (PharmacoMIP) network 2-year pilot study. Drug Safety 2010;33(5):409-16. - PubMed
Haramburu 1988 {published data only}
-
- Haramburu F, Pere JC, Begaud B, Albin H. Has mandatory reporting changed the rate of adverse drug reaction spontaneous reports? [Declaration obligatoire des effets indesirables des medicaments: le decret a-t-il modifie la notification spontanee?]. Therapie 1988;43(6):493-6. - PubMed
Haw 2011 {published data only}
-
- Haw C, Cahill C. A computerized system for reporting medication events in psychiatry: the first two years of operation. Journal of Psychiatric and Mental Health Nursing 2011;18(4):308-15. - PubMed
He 2018 {published data only}
Iessa 2021 {published data only}
Jacqout 2012 {published data only}
-
- Jacquot J, Gony M, Baudrin D, Chastel X, Montastruc JL, Bagheri H. Could we improve notification of adverse drugs reactions in hospital? Assessment of 5 years of network PharmacoMIP's activities [Peut-on ameliorer la notification des effets indesirables dans les etablissements hospitaliers? Bilan de 5 ans d'activite du reseau PharmacoMIP]. Therapie 2012;67(3):231-6. - PubMed
Jha 2017 {published data only}
-
- Jha N, Rathore DS, Shankar PR, Bhandary S, Alshakka M, Gyawali S. Knowledge, attitude and practice regarding pharmacovigilance and consumer pharmacovigilance among consumers at Lalitpur District, Nepal. Journal of Nepal Health Research Council 2017;15(1):31-7. - PubMed
Kronenfeld 2019 {published data only}
Lata 2004 {published data only}
-
- Lata PF, Mainhardt M, Johnson CA. Impact of nurse case manager-pharmacist collaboration on adverse-drug-event reporting. American Journal of Health-System Pharmacy 2004;61(5):483-7. - PubMed
Lee 2004 {published data only}
-
- Lee, S B, Schepers, G P, Goldberg, K L. Electronic adverse drug reaction reporting program. Am J Health Syst Pharm 2004;61(12):1230-1233. - PubMed
Li 2020 {published data only}
-
- Li, R, Curtis, K, Zaidi, S T R, Van, C, Castelino, R. Effect of the black triangle scheme and its online educational campaign on the quantity and quality of adverse drug event reporting in Australia: a time series analysis. Expert Opin Drug Saf 2020;19(6):747-753. - PubMed
Linder 2010 {published data only}
-
- Linder, J A, Haas, J S, Iyer, A, Labuzetta, M A, Ibara, M, Celeste, M, Getty, G, Bates, D W. Secondary use of electronic health record data: spontaneous triggered adverse drug event reporting. Pharmacoepidemiol Drug Saf 2010;19(12):1211-1215. - PubMed
Lynn 2010 {published data only}
-
- Lynn, R M, Riding, K, McIntosh, N. The use of electronic reporting to aid surveillance of ADRs in children: a proof of concept study. Arch Dis Child 2010;95(4):262-265. - PubMed
Marquez 2016 {published data only}
-
- Marquez ST, Herdeiro MT, Vaz IR. An educational intervention to improve nurses reporting of adverse drug reactions. Pharmacoepidemiology and Drug Safety 2016;25:403. [DOI: ]
-
- Marquez, S, Herdeiro, MT, Ribeiro-Vaz, I. An Educational Intervention to improve nurses reporting of Adverse drug reactions. Clinical Therapeutics 2015;37:e57.
Massah 2021 {published data only}
McGettigan 1997 {published data only}
Menat 2021 {published data only}
Morales 2016 {published data only}
-
- Morales Rios O, Jasso Gutierrez L, Talavera JO, Tellez-Rojo MM, Olivar Lopez V, Garduno Espinosa J, etal. A comprehensive intervention for adverse drug reactions identification and reporting in a Pediatric Emergency Department. International Journal of Clinical Pharmacy 2016;38(1):80-7. - PubMed
Morgan 1990 {published data only}
-
- Morgan SA, Frank JT. Development of a videotape on adverse drug reactions. American Journal of Hospital Pharmacy 1990;47(6):1340-1342. - PubMed
Mwamwitwa 2022 {published data only}
-
- Mwamwitwa KW, Fimbo AM, Bukundi EM, Nkayamba AF, Buma D, Muro EP, Maganda BA, Shewiyo DH, Shearer MC, Smith AD, Kaale EA. Effectiveness of a structured stimulated spontaneous safety monitoring of medicines reporting program in strengthening pharmacovigilance system in Tanzania. Sci 2022;12(1):16131. - PMC - PubMed
NCT02087293 {published data only}
-
- NCT02087293. e-Pharmacovigilance II - surveillance for safety and effectiveness - Calling for Earlier Detection of Adverse Reactions (CEDAR). https://ClinicalTrials.gov/show/NCT02087293 (first received 11 March 2014).
Ng 2020 {published data only}
-
- Ng TM, Teo CJ, Heng ST, Chen YR, Lim WP, Teng CB. Impact of round-the-clock pharmacist inpatient medication chart review on medication errors. JACCP Journal of the American College of Clinical Pharmacy 2020;3(8):1437-43.
Opadeyi 2019 {published data only}
Ortega 2008 {published data only}
-
- Ortega A, Aguinagalde A, Lacasa C, Aquerreta I, Fernández-Benítez M, Fernández LM. Efficacy of an adverse drug reaction electronic reporting system integrated into a hospital information system. Annals of Pharmacotherapy 2008;42:1491-6. - PubMed
Potlog 2020 {published data only}
Praveen 2021 {published data only}
-
- Praveen KS, Ramesh B, Sachin PR, Mubashir A. Impact of an awareness program on adverse drug reaction reporting among community pharmacist. European Journal of Clinical Pharmacy 2021;23(2):133-8.
Reumerman 2021a {published data only}
-
- Reumerman M, Tichelaar J, Van Eekeren R, Van Puijenbroek EP, Richir MC, Van Agtmael MA. The potential of training specialist oncology nurses in real-life reporting of adverse drug reactions. European Journal of Clinical Pharmacology 2021;77(10):1531-42. [DOI: 10.1007/s00228-021-03138-5] - DOI - PMC - PubMed
Reumerman 2021b {published data only}
Rogers 1989 {published data only}
-
- Rogers SA. Strategies to promote physician reporting of adverse drug events based on the experience of an FDA-sponsored project in Maryland. Journal of Clinical Research and Drug Development 1989;3(1):29-37.
Roy 2018 {published data only}
Rubin 2019 {published data only}
-
- Rubin DS, Pesyna C, Jakubczyk S, Liao C, Tung A. Introduction of a mobile adverse event reporting system Is associated with participation in adverse event reporting. American Journal of Medical Quality 2019;34(1):30-5. - PubMed
Salcedo de Diego 2015 {published data only}
-
- Salcedo de Diego I Serrano Gallardo P, Ruiz Antorán B, De Andrés Gimeno B, Revuelta Zamorano M, Avendaño Solá C. Impact of a multicomponent intervention on registered nurses to increase reporting of adverse drug reactions and medication errors. Clinical Therapeutics 2015;37(8):e153.
Sánchez 2014 {published data only}
-
- Sánchez I, Amador C, Plaza JC, Correa G, Amador R. Assessment of an active pharmacovigilance system carried out by a pharmacist. Revista Médica de Chile 2014;142(8):998-1005. - PubMed
Sanko 2020 {published data only (unpublished sought but not used)}
-
- Sanko JS, McKay M. Participation in a system-thinking simulation experience changes adverse event reporting. Simulation in Healthcare 2020;15(3):167-71. - PubMed
Schindler 2019 {published data only}
-
- Schindler TM, Bridge H. For safety’s sake part 2: reporting of adverse event data. AMWA Journal 2019;34(1):32-7.
Segec 2021 {published data only}
Sonowa 2020 {published data only}
-
- Sonowa S, Desai CK, Panchal JR. Impact of certain educational interventions on adverse drug reaction reporting by nursing health professionals at a tertiary care hospital. Asian Journal of Pharmaceutical and Clinical Research 2020;13(6):175-80.
Srikanth 2019 {published data only}
-
- Srikanth MS, Adepu R. Assessment of structured educational intervention and barriers of community pharmacists towards adverse drug reaction reporting and monitoring in South Indian district. Drug Invention Today 2019;12:131-41.
Tabali 2009 {published data only}
Terblanche 2018 {published data only}
-
- Terblanche A, Meyer JC, Godman B, Summers RS. Impact of a pharmacist-driven pharmacovigilance system in a secondary hospital in the Gauteng Province of South Africa. Hospital Practice (1995) 2018;46(4):221-8. - PubMed
Touchette 2012 {published data only}
-
- Touchette DR, Masica AL, Dolor RJ, Schumock GT, Choi YK, Kim Y. Safety-focused medication therapy management: a randomized controlled trial. Journal of the American Pharmacists Association: JAPhA 2012;52(5):603-12. - PubMed
Varallo 2015 {published data only}
-
- Varallo FR. Educational intervention in pharmacovigilance for hospital health professionals. https://ClinicalTrials.gov/show/NCT02134587 (first received 5 May 2014). [NCT02134587]
Weant 2010 {published data only}
-
- Weant KA, Humphries RL, Hite K, Armitstead JA. Effect of emergency medicine pharmacists on medication-error reporting in an emergency department. American Journal of Health-System Pharmacy 2010;67(21):1851-5. - PubMed
Welsh 1996 {published data only}
-
- Welsh CH, Pedot R, Anderson RJ. Use of morning report to enhance adverse event detection. Journal of General Internal Medicine 1996;11(8):454-60. - PubMed
Wengrove 2021 {published data only}
-
- Wengrove McCracken A, Grimes M, Sherman B, Decker K, Hirsch D, Pham A, et al. Implementation of standard nursing workflow for adverse drug reaction reporting within health system ambulatory infusion services. Infusion 2021;27(1):12-7.
Wentzell 2017 {published data only}
Winstanley 1989 {published data only}
Xu 2014 {published data only}
-
- Xu C, Li G, Ye N, Lu Y. An intervention to improve inpatient medication management: a before and after study. Journal of Nursing Management 2014;22(3):286-94. - PubMed
Yen 2010 {published data only}
-
- Yen Y-H, Kuo L-N, Hsu M-H, Li Y-C, Cheng K-J. Evaluation of the electronic adverse drug event management system. Journal of Experimental & Clinical Medicine 2010;2(6):287-91.
References to studies awaiting assessment
Biagi 2013 {published data only}
Kim 2012 {published data only}
-
- Kim M, McPherson ML, Smith SW, Kopochis E. Impact of an electronic medication error reporting system in a hospice organization. Journal of Pain and Symptom Management 2012;43(2):449. [DOI: ]
Kumar 2021 {published data only}
-
- Acceptability and feasibility of mobile app in adverse drug reactions (ADRs) reporting in Revised National Tuberculosis Control Program (RNTCP) centres. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/07/035163 (first received 27 July 2021).
Lan 2022 {published data only}
Lander 2013 {published data only}
-
- Lander AR, Blicher TM, Jimenez-Solem E, Jespersen M, Kampmann JP, Christensen HR. Introducing an adverse drug event manager. European Journal of Hospital Pharmacy: Science and Practice 2013;20:78-81.
Marquez 2015 {published data only}
-
- Marquez S, Herdeiro MT, Ribeiro-Vaz I. An educational intervention to improve nurses reporting of adverse drug reactions. Clinical Therapeutics 2015;37(8):e57.
Opadeyi 2021 {published data only}
-
- Opadeyi AO, Fourrier-Reglat A, Isah AO. Impact of an educational intervention on adverse drug reaction reporting in tertiary hospitals in South-South Nigeria. West African Journal of Medicine 2021;38(7):634-45. - PubMed
Sawangjit 2022 {published data only}
-
- Effectiveness of TaWai mobile system in Lao version: a cluster randomized controlled trial in Lao, PDR. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220607002 (first received 7 June 2022).
References to ongoing studies
Hutchinson 2020 {published data only}
-
- Hutchinson AM, Brotto V, Chapman A, Sales AE, Mohebbi M, Bucknall TK. Use of an audit with feedback implementation strategy to promote medication error reporting by nurses. Journal of Clinical Nursing 2020;29(21-2):4180-93. - PubMed
Kiguba 2022 {published data only}
-
- Correction: Effectiveness of the med Safety mobile application in improving adverse drug reaction reporting by healthcare professionals in Uganda: a protocol for a pragmatic cluster-randomised controlled trial. BMJ Open 2024;14:e061725corr1. [DOI: 10.1136/bmjopen-2022-061725corr1] - DOI - PMC - PubMed
-
- Kiguba R, Mwebaza N, Ssenyonga R, Ndagije HB, Nambasa V, Katureebe C, et al. Effectiveness of the Med Safety mobile application in improving adverse drug reaction reporting by healthcare professionals in Uganda: a protocol for a pragmatic cluster-randomised controlled trial. BMJ Open 2022;12(7):e061725. - PMC - PubMed
NCT05402254 {published data only}
-
- NCT05402254. Impact of a pharmacovigilance program led by advanced practice nursing (IMPACTO). https://www.clinicaltrials.gov/study/NCT05402254 (first received 23 May 2022).
Additional references
Abstoss 2011
-
- Abstoss KM, Shaw BE, Owens TA, Juno JL, Commiskey EL, Niedner MF. Increasing medication error reporting rates while reducing harm through simultaneous cultural and system-level interventions in an intensive care unit. BMJ Quality and Safety 2011;20(11):914-22. - PubMed
Al Hamid 2014
Andel 2012
-
- Andel C, Davidow SL, Hollander M, Moreno DA. The economics of health care quality and medical errors. Journal of Health Care Finance 2012;39(1):39. - PubMed
Classen 1997
-
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. Journal of the American Medical Association 1997;277(4):301-6. - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed prior to 22 October 2024. Melbourne, Australia: Veritas Health Innovation, 2024. Available at https://www.covidence.org.
Cumby 1990
-
- Cumby RE, Huizinga J. Testing the autocorrelation structure of disturbances in ordinary least squares and instrumental variables regressions. Econometrica 1992;60(1):185-95.
EMA 2016
-
- European Medicines Agency (EMA). Good pharmacovigilance practices. www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/d.... Accessed 2 October 2016.
EndNote 2013 [Computer program]
-
- EndNote. Version X4. Thomson Reuters, 2013.
EPOC 2013a
-
- Effective Practice and Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. epoc.cochrane.org/epoc-specific-resources-review-authors Accessed prior to 28 February 2017.
EPOC 2013b
-
- Effective Practice and Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. epoc.cochrane.org/epoc-specific-resources-review-authors Accessed prior to 28 February 2017.
EPOC 2013c
-
- Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC resources for review authors. Oslo: Norwegian Knowledge Center for the Health Services; 2013. epoc.cochrane.org/epoc-specific-resources-review-authors Accessed prior to 28 February 2017.
EPOC 2013d
-
- Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. epoc.cochrane.org/epoc-specific-resources-review-authors Accessed prior to 28 February 2017.
EPOC 2015b
-
- Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015. epoc.cochrane.org/epoc-specific-resources-review-authors Accessed prior to 28 February 2017.
Ernst 2001
-
- Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. Journal of the American Pharmaceutical Association 2001;41(2):192-199. - PubMed
Feely 1990
Figueiras 2001
-
- Figueiras A, Tato F, Fontaiñas J, Takkouche B, Gestal-Otero JJ. Physicians’ attitudes towards voluntary reporting of adverse drug events. Journal of Evaluation in Clinical Practice 2001;7(4):347-54. - PubMed
Fornasier 2018
Gad 2009
-
- Gad SC. Postmarketing safety evaluation: monitoring, assessing, and reporting of adverse drug responses. In: Drug Safety Evaluation. Second edition. Hoboken, New Jersey: John Wiley & Sons, 2009:943-44.
Gonzalez‐Gonzalez 2013
-
- Gonzalez-Gonzalez C, Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Strategies to improve adverse drug reactions reporting: a critical and systematic review. Drug Safety 2013;36(5):317-28. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT. Version Accessed prior to 28 February 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal 2008;336(7650):924-6. - PMC - PubMed
Gyllensten 2013
Hazell 2006
-
- Hazell L, Shakir SAW. Under reporting of adverse drug reactions: a systematic review. Drug Safety 2006;29(5):385-96. - PubMed
Higgins 2024
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Cochrane 2024:Available from www.training.cochrane.org/handbook.
Johnson 1995
-
- Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Archives of Internal Medicine 1995;155(18):1949-56. - PubMed
Khalili 2020
-
- Khalili M, Mesgarpour B, Sharifi H, Daneshvar Dehnavi S, Haghdoost AA. Interventions to improve adverse drug reaction reporting: a scoping review. Pharmacoepidemiology and Drug Safety 2020;29(9):965-92. - PubMed
Kunac 2014
-
- Kunac DL, Tatley MV, Seddon ME. A new web-based Medication Error Reporting Programme (MERP) to supplement pharmacovigilance in New Zealand--findings from a pilot study in primary care. New Zealand Medical Journal 2014;127(1401):69-81. - PubMed
Lazarou 1998
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. Journal of the American Medical Association 1998;279(15):1200-5. - PubMed
Li 2019
-
- Li R, Zaidi STR, Chen T, Castelino R. Effectiveness of interventions to improve adverse drug reaction reporting by healthcare professionals over the last decade: a systematic review. Pharmacoepidemiology and Drug Safety 2019;29:1-8. - PubMed
Liberati 2009
Linden 2015
-
- Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata Journal 2015;15(2):480-500.
Lindquist 2008
-
- Lindquist M. The WHO Global ICSR database system: basic facts. Drug Information Journal 2008;42:409-19.
McGettigan 1997
Mirbaha 2015
Molokhia 2009
Moride 1997
NCCMERP 2016
-
- National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP). What is a medication error? www.nccmerp.org. Accessed 28 February 2024.
O'Brien 2007
-
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard‐Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD000409. [DOI: 10.1002/14651858.CD000409.pub2] - DOI - PMC - PubMed
Ortega 2008
-
- Ortega A, Aguinagalde A, Lacasa C, Aquerreta I, Fernández-Benítez M, Fernández LM. Efficacy of an adverse drug reaction electronic reporting system integrated into a hospital information system. Annals of Pharmacotherapy 2008;42(10):1491-6. - PubMed
Pagotto 2013
-
- Pagotto C, Varallo F, Mastroianni F. Impact of educational interventions on adverse drug events reporting. International Journal of Technology Assessment in Health Care 2013;29(4):410-17. - PubMed
Pal 2013
Paudyal 2020
-
- Paudyal V, Al-Hamida A, Bowena M, Abdul Hadi M, Hasan SS, Jalala Z, Stewart D. Interventions to improve spontaneous adverse drug reaction reporting by healthcare professionals and patients: systematic review and meta-analysis. Expert Opinion on Drug Safety 2020;19(9):1173–91. - PubMed
Pirmohamed 2004
Ribeiro‐Vaz 2016
Rücker 2017
-
- Rücker G, Cates CJ, G Schwarzer. Methods for including information from multi-arm trials in pairwise meta-analysis. Research Synthesis Methods 2017;8(4):392-403. - PubMed
Shalviri 2009
-
- Shalviri G, Valadkhani M, Dinarvand R. Ten years pharmacovigilance activities in Iran. Iranian Journal of Public Health 2009;38(Suppl 1):162-5.
Shalviri 2012
-
- Shalviri G, Yousefian S, Gholami K. Adverse events induced by ceftriaxone: a 10-year review of reported cases to Iranian Pharmacovigilance Centre. Journal of Clinical Pharmacy and Therapeutics 2012;37(4):448-51. - PubMed
Sterne 2011
Uppsala Monitoring Center
-
- Uppsala Monitoring Center. Glossary of terms used in pharmacovigilance. www.who-umc.org. Accessed 28 January 2024.
Varallo 2014
-
- Varallo FR, Guimarães S de O, Abjaude SA, Mastroianni P de C. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Revista da Escola de Enfermagem da USP 2014;48(4):739-47. - PubMed
Wester 2008
WHO 2006
-
- World Health Organization. The safety of medicines in public health programmes:pharmacovigilance an essential tool. www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharmacovigil... Accessed 2 October 2016. [ISBN 92 4 159391 1]
WHO 2014
-
- World Health Organization. Reporting and learning systems for medication errors: the role of pharmacovigilance centres. apps.who.int/iris/bitstream/10665/137036/1/9789241507943_eng.pdf?ua=1 Accessed prior to 28 February 2017. [ISBN 978 92 4 150794 3]
WHO 2016
-
- World Health Organization. Annual meeting of representatives of National Pharmacovigilance Centres participating in the WHO Programme for International Drug Monitoring. www.who.int//medicines/regulation/medicines-safety/npvc-meeting/en/ Accessed 2 October 2016.
Wilson 2012
-
- Wilson RM, Michel P, Olsen S, Gibberd RW, Vincent C, El-Assady R, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. British Medical Journal 2012;344:e832. [DOI: ] - PubMed
World Bank
-
- World Bank. World Development Indicators (GDP). http://data.worldbank.org/indicator/NY.GDP.MKTP.CD 2014.
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

