Clinical validation of an RSV neutralization assay and analysis of cross-sectional sera associated with 2021-2023 RSV outbreaks to investigate the immunity debt hypothesis
- PMID: 39470275
- PMCID: PMC11619414
- DOI: 10.1128/spectrum.02115-24
Clinical validation of an RSV neutralization assay and analysis of cross-sectional sera associated with 2021-2023 RSV outbreaks to investigate the immunity debt hypothesis
Abstract
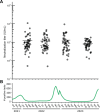
Respiratory syncytial virus (RSV) is a leading cause of acute respiratory infections and hospitalization in infants and the elderly. Newly approved vaccines and the prophylactic antibody nirsevimab have heightened interest in RSV immunologic surveillance, necessitating the development of high-throughput assays assessing anti-RSV neutralizing activity. Quantitative viral neutralization remains the best correlate of protection for RSV infection and the gold standard for RSV immunological testing. Here, we developed a high-throughput RSV strain A2 focus-reduction neutralization test validated to Clinical Laboratory Improvement Amendments (CLIA)/ Good Clinical Laboratory Practices (GCLP) standards using both clinical specimens and commercially available reference sera. The assay is highly accurate, generating reference serum neutralizing titers within twofold of established assays, with an analytical measurement range between 8 and 1,798 international units per mL (IU/mL). Neutralizing activity measured by the assay strongly correlated with antibody titer determined via indirect enzyme-linked immunosorbent assay (ELISA) (ρ = 1.0, P = 0.0014). Individuals recently having tested positive via quantitative reverse transcription polymerase chain reaction (RT-qPCR) for RSV had a 9.1-fold higher geometric mean neutralizing titer relative to RSV PCR negatives (P-value = 0.09). The validated assay was then used to investigate the immunity debt hypothesis for resurgent RSV outbreaks in the 2022-2023 season, using adult clinical remnant sera sent for herpes simplex virus (HSV)-1/2 antibody testing. There was no difference in geometric mean anti-RSV neutralizing titers between sera sampled before and after the 2022-2023 RSV outbreak (P = 0.68). These data are consistent with limited changes in RSV-neutralizing antibody levels in adults across the 2022-23 RSV outbreak.
Importance: Population surveillance studies of serum-neutralizing activity against RSV are crucial for evaluating RSV vaccine efficacy and vulnerabilities to new strains. Here, we designed and validated a high-throughput assay for assessing anti-RSV neutralizing activity, standardized its measurements for comparison with other methodologies, and demonstrated its applicability to real-world samples. Our assay is precise, linear, and yields measurements consistent with other standardized assays, offering a methodology useful for large-scale studies of RSV immunity. We also find no significant difference in neutralizing titers among adults between those taken before and after large RSV outbreaks associated with the latter stages of the coronavirus disease of 2019 (COVID-19) public health emergency, underlining the need for a greater understanding of the dynamics of serological responses to RSV infection.
Keywords: IU/mL; RSV; correlate of protection; epidemiology; neutralization; outbreaks; seroepidemiology; serology; titer; validation.
Conflict of interest statement
ALG reports contract testing from Abbott, Novavax, Cepheid, Pfizer, Janssen and Hologic and research support from Gilead, outside of the described work.
Figures


References
-
- Respiratory Syncytial Virus (RSV) | NIH: National Institute of Allergy and Infectious Diseases. 2022. Available from: https://www.niaid.nih.gov/diseases-conditions/respiratory-syncytial-viru.... Retrieved 10 Jun 2023.
-
- Commissioner O of the . 2023. FDA approves first respiratory syncytial virus (RSV) vaccine. FDA. FDA. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-r...
LinkOut - more resources
Full Text Sources

