Quantitating SARS-CoV-2 neutralizing antibodies from human dried blood spots
- PMID: 39470282
- PMCID: PMC11619372
- DOI: 10.1128/spectrum.00846-24
Quantitating SARS-CoV-2 neutralizing antibodies from human dried blood spots
Abstract
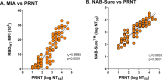
In the earliest days of the COVID-19 pandemic, the collection of dried blood spots (DBS) enabled public health laboratories to undertake population-scale seroprevalence studies used to estimate rates of SARS-CoV-2 exposure. With SARS-CoV-2 seropositivity levels now estimated to exceed 94% in the United States, attention has turned to using DBS to assess neutralizing antibodies within cohorts of interest. With this goal in mind, we generated contrived DBS (cDBS) and whole blood-derived DBS from convalescent and vaccinated individuals and subjected DBS eluates to a battery of assays, including a SARS-CoV-2 multiplexed microsphere immunoassay (MIA), a receptor binding domain (RBD)-human ACE2 inhibition assay (iACE2), a cell-based pseudovirus neutralization assay, and real-time PCR-based surrogate neutralization assay (NAB-Sure). The DBS results were benchmarked against paired serum samples tested in a clinically validated SARS-CoV-2 plaque reduction neutralization titer (PRNT) assay. The results of an 8-plex MIA and NAB-Sure assays demonstrated highly significant correlations with PRNT values when evaluated with a panel of 86 paired serum-DBS samples. Both the MIA and NAB-Sure are adaptable to automated liquid handlers for high-throughput capacity. While neutralizing assays were limited to the ancestral SARS-CoV-2 WA1, this study nonetheless represents an important proof of concept demonstrating the potential utility of DBS as a biospecimen type for use in assessing immunity to SARS-CoV-2 at the community and population levels.IMPORTANCESARS-CoV-2 variants of concern continue to circulate globally and remain a serious health threat to large segments of the population. From a public health standpoint, identifying vulnerable communities based on immune status is critical in terms of vaccine booster recommendations. In this report, we investigated the utility of dried blood spots (DBS) as a biospecimen type from which to estimate SARS-CoV-2 neutralizing antibody titers. Using contrived and whole blood-derived DBS, we demonstrate that SARS-CoV-2 neutralizing antibodies are readily measurable in DBS eluates and correlate with plaque reduction neutralization titer (PRNT) values from paired serum samples. Moreover, several of the methods used to estimate SARS-CoV-2 neutralizing antibodies in DBS eluates are adaptable to high-throughput platforms.
Keywords: COVID-19; antibody; human; neutralizing; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Update of
-
Quantitating SARS-CoV-2 Neutralizing Antibodies from Human Dried Blood Spots.bioRxiv [Preprint]. 2024 Apr 2:2024.03.18.585599. doi: 10.1101/2024.03.18.585599. bioRxiv. 2024. Update in: Microbiol Spectr. 2024 Oct 29:e0084624. doi: 10.1128/spectrum.00846-24. PMID: 38562708 Free PMC article. Updated. Preprint.
References
-
- Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, Parker MM, John Leung SY, Morne JE, Greene D, Holtgrave DR, Hoefer D, Kumar J, Udo T, Hutton B, Zucker HA. 2020. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol 48:23–29. doi:10.1016/j.annepidem.2020.06.004 - DOI - PMC - PubMed
-
- Damjanovic A, Styer LM, Nemeth K, Yauney E, Rock JM, Bievenue R, Hoen R, Ehrbar D, Kay DM, Caggana M, Parker MM. 2022. Utility of newborn dried blood spots to ascertain seroprevalence of SARS-CoV-2 antibodies among individuals giving birth in New York State, November 2019 to November 2021. JAMA Netw Open 5:e2227995. doi:10.1001/jamanetworkopen.2022.27995 - DOI - PMC - PubMed
-
- Amendola A, Bianchi S, Gori M, Barcellini L, Colzani D, Canuti M, Giacomet V, Fabiano V, Folgori L, Zuccotti GV, Tanzi E. 2021. Dried blood spot as an alternative to plasma/serum for SARS-CoV-2 IgG detection, an opportunity to be sized to facilitate COVID-19 Surveillance among schoolchildren. Pediatr Infect Dis J 40:e46–e47. doi:10.1097/INF.0000000000002955 - DOI - PubMed
-
- Basto-Abreu A, Carnalla M, Torres-Ibarra L, Sanchez-Pájaro A, Romero-Martínez M, Martínez-Barnetche J, López-Martínez I, Aparicio-Antonio R, Shamah-Levy T, Alpuche-Aranda C, Rivera JA, Barrientos-Gutiérrez T, Ensanut-Covid Collaborators . 2023. SARS-CoV-2 seroprevalence and vaccine coverage from august to november 2021: a nationally representative survey in Mexico. J Med Virol 95:e29038. doi:10.1002/jmv.29038 - DOI - PubMed
-
- Matias WR, Fulcher IR, Sauer SM, Nolan CP, Guillaume Y, Zhu J, Molano FJ, Uceta E, Collins S, Slater DM, Sanchez VM, Moheed S, Harris JB, Charles RC, Paxton RM, Gonsalves SF, Franke MF, Ivers LC. 2023. Disparities in SARS-CoV-2 infection by race, ethnicity, language, and social vulnerability: evidence from a citywide seroprevalence study in Massachusetts, USA. J Racial Ethn Health Disparities. doi:10.1007/s40615-022-01502-4:1-11 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

