Imaging PD-L1 in the brain-Journey from the lab to the clinic
- PMID: 39470381
- PMCID: PMC11812043
- DOI: 10.1093/neuonc/noae190
Imaging PD-L1 in the brain-Journey from the lab to the clinic
Abstract
Background: Immune checkpoint inhibitors (ICPIs) have proven to restore adaptive anti-tumor immunity in many cancers; however, no noteworthy therapeutic schedule has been established for patients with glioblastoma (GBM). High programmed death-ligand 1 (PD-L1) expression is associated with immunosuppressive and aggressive phenotypes in GBM. Presently, there is no standardized protocol for assessing PD-L1 expression levels to select patients and monitor their response to ICPI therapy. The aim of this study was to investigate the use of 89Zr-DFO-Atezolizumab to image the spatio-temporal distribution of PD-L1 in preclinical mouse models and in patients with newly diagnosed GBM treated with/without neoadjuvant Pembrolizumab.
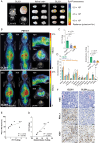
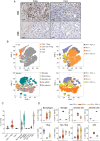
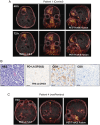
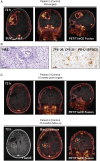
Methods: The immunoreactivity, binding affinity, and specificity of 89Zr-DFO-Atezolizumab were confirmed in vitro. Mice-bearing orthotopic GBM tumors or patients with newly diagnosed GBM treated with/without Pembrolizumab were intravenously injected with 89Zr-DFO-Atezolizumab, and PET/CT images were acquired 24, 48, and 72 hours in mice and at 48 and 72 post-injection in patients. Radioconjugate uptake was quantified in the tumor and healthy tissues. Ex vivo immunohistochemistry (IHC) and immunophenotyping were performed on mouse tumor samples or resected human tumors.
Results: 89Zr-DFO-Atezolizumab was prepared with high radiochemical purity (RCP > 99%). In vitro cell-associated radioactivity of 89Zr-DFO-Atezolizumab corroborated cell line PD-L1 expression. PD-L1 in mouse GBM tumors was detected with high specificity using 89Zr-DFO-Atezolizumab and radioconjugate uptake correlated with IHC. Patients experienced no 89Zr-DFO-Atezolizumab-related side effects. High 89Zr-DFO-Atezolizumab uptake was observed in patient tumors at 48 hours post-injection, however, the uptake varied between patients treated with/without Pembrolizumab.
Conclusions: 89Zr-DFO-Atezolizumab can visualize distinct PD-L1 expression levels with high specificity in preclinical mouse models and in patients with GBM, whilst complementing ex vivo analysis.
Keywords: glioblastoma; immuno-PET; immunotherapy; nuclear medicine; translational studies.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Wen PY, Reardon DA.. Neuro-oncology in 2015: Progress in glioma diagnosis, classification and treatment. Nat Rev Neurol. 2016;12(2):69–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

