Efficacy and safety of thalidomide in patients with β-thalassemia intermedia and major
- PMID: 39470504
- PMCID: PMC11521010
- DOI: 10.1097/MD.0000000000040328
Efficacy and safety of thalidomide in patients with β-thalassemia intermedia and major
Abstract
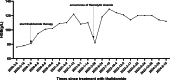
To investigate the short-term and long-term efficacy and safety of thalidomide in the treatment of intermediate and severe β-thalassemia. We analyzed patients with intermediate and severe β-thalassemia treated at our hospital from February 2019 to February 2024. Patients who received treatment for more than 3 months were included. The efficacy of thalidomide was assessed by comparing changes in hemoglobin (Hb), ferritin, bilirubin, and Hb electrophoresis before and after treatment. Adverse drug reactions during treatment were also recorded. A total of 42 β-thalassemia patients were included, with thalidomide dosages ranging from 75 to 150 mg/d. The response rates at 1, 3, and 6 months of treatment were 73.8% (31/42), 75.0% (24/32), and 94.7% (18/19), respectively. The increase in Hb levels was primarily attributed to fetal hemoglobin (HbF). After 1 month of treatment, the HbF percentage increased from a baseline of 34.04 ± 27.58% to 56.25 ± 28.40% (P < .001). At 3 and 6 months, HbF further increased to 67.21 ± 27.12% (P < .001) and 73.93 ± 22.96% (P < .001), respectively. The average duration of thalidomide treatment was 25.3 ± 9.2 months (range: 4-60 months), with 6 patients treated for over 60 months and 18 patients for over 48 months. Two homozygous patients who failed thalidomide treatment achieved Hb levels above 100 g/L and discontinued transfusion therapy after 3 months of hydroxyurea treatment. The most common adverse reaction was somnolence, which was mild and tolerable. Thalidomide demonstrates significant and sustained therapeutic effects in β-thalassemia patients. The adverse reactions are mild and tolerable, allowing patients to continue treatment.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Clinical efficacy of thalidomide for various genotypes of beta thalassemia.BMC Med Genomics. 2024 Jul 18;17(1):191. doi: 10.1186/s12920-024-01963-y. BMC Med Genomics. 2024. PMID: 39026312 Free PMC article.
-
Clinical trial on the effects of thalidomide on hemoglobin synthesis in patients with moderate thalassemia intermedia.Ann Hematol. 2018 Oct;97(10):1933-1939. doi: 10.1007/s00277-018-3395-5. Epub 2018 Jun 22. Ann Hematol. 2018. PMID: 29931453 Clinical Trial.
-
Comparison of efficacy and safety of thalidomide vs hydroxyurea in patients with Hb E-β thalassemia - a pilot study from a tertiary care Centre of India.Blood Cells Mol Dis. 2021 May;88:102544. doi: 10.1016/j.bcmd.2021.102544. Epub 2021 Feb 3. Blood Cells Mol Dis. 2021. PMID: 33610115
-
Hydroxyurea-induced hematological response in transfusion-independent beta-thalassemia intermedia: case series and review of literature.Pediatr Hematol Oncol. 2009 Nov;26(8):560-5. doi: 10.3109/08880010903271671. Pediatr Hematol Oncol. 2009. PMID: 19954365 Review.
-
Investigating the Efficacy and Safety of Thalidomide for Treating Patients With ß-Thalassemia: A Meta-Analysis.Front Pharmacol. 2022 Jan 11;12:814302. doi: 10.3389/fphar.2021.814302. eCollection 2021. Front Pharmacol. 2022. PMID: 35087410 Free PMC article. Review.
References
-
- Shang X, Wu X, Zhang X, Feng X, Xu X; Writing Group For Practice Guidelines For Diagnosis And Treatment Of Genetic Diseases Medical Genetics Branch Of Chinese Medical Association. Clinical practice guidelines for beta-thalassemia. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2020;37:243–51. - PubMed
-
- Rani S, Gupta A, Bhardwaj M. Epidermolysis bullosa pruriginosa: a rare entity which responded well to thalidomide. Dermatol Ther. 2019;32:e13035. - PubMed
-
- Shah FT, Sayani F, Trompeter S, Drasar E, Piga A. Challenges of blood transfusions in beta-thalassemia. Blood Rev. 2019;37:100588. - PubMed
-
- Cappellini MD, Porter JB, Viprakasit V, Taher AT. A paradigm shift on beta-thalassaemia treatment: how will we manage this old disease with new therapies? Blood Rev. 2018;32:300–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

