Suppression of melanoma by mice lacking MHC-II: Mechanisms and implications for cancer immunotherapy
- PMID: 39470607
- PMCID: PMC11528124
- DOI: 10.1084/jem.20240797
Suppression of melanoma by mice lacking MHC-II: Mechanisms and implications for cancer immunotherapy
Abstract
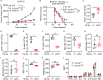
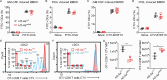
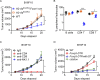
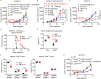
Immune checkpoint inhibitors interfere with T cell exhaustion but often fail to cure or control cancer long-term in patients. Using a genetic screen in C57BL/6J mice, we discovered a mutation in host H2-Aa that caused strong immune-mediated resistance to mouse melanomas. H2-Aa encodes an MHC class II α chain, and its absence in C57BL/6J mice eliminates all MHC-II expression. H2-Aa deficiency, specifically in dendritic cells (DC), led to a quantitative increase in type 2 conventional DC (cDC2) and a decrease in cDC1. H2-Aa-deficient cDC2, but not cDC1, were essential for melanoma suppression and effectively cross-primed and recruited CD8 T cells into tumors. Lack of T regulatory cells, also observed in H2-Aa deficiency, contributed to melanoma suppression. Acute disruption of H2-Aa was therapeutic in melanoma-bearing mice, particularly when combined with checkpoint inhibition, which had no therapeutic effect by itself. Our findings suggest that inhibiting MHC-II may be an effective immunotherapeutic approach to enhance immune responses to cancer.
© 2024 Shi et al.
Conflict of interest statement
Disclosures: B. Beutler reported support from Pfizer Inc. during the conduct of the study; in addition, B. Beutler and H. Shi had U.S. Provisional Patent Application Serial No. 62/702,088 pending. No other disclosures were reported.
Figures









References
-
- Binnewies, M., Mujal A.M., Pollack J.L., Combes A.J., Hardison E.A., Barry K.C., Tsui J., Ruhland M.K., Kersten K., Abushawish M.A., et al. 2019. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell. 177:556–571.e16. 10.1016/j.cell.2019.02.005 - DOI - PMC - PubMed
-
- Bosteels, C., Neyt K., Vanheerswynghels M., van Helden M.J., Sichien D., Debeuf N., De Prijck S., Bosteels V., Vandamme N., Martens L., et al. 2020. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity. 52:1039–1056.e9. 10.1016/j.immuni.2020.04.005 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

