BRIVA-ONE study: 12-month outcomes of brivaracetam monotherapy in clinical practice
- PMID: 39470722
- PMCID: PMC11633701
- DOI: 10.1002/epi4.13078
BRIVA-ONE study: 12-month outcomes of brivaracetam monotherapy in clinical practice
Abstract
Objective: This study investigated the effectiveness and tolerability of brivaracetam (BRV) monotherapy in a large series of patients with epilepsy.
Method: This was a multicenter, retrospective, observational, non-interventional study in 24 hospitals across Spain. Patients aged ≥18 years who started on BRV monotherapy, either as first-line or following conversion, at least 1 year before database closure were included. Patients were evaluated at baseline and at 3, 6 and 12 months after initiation of BRV monotherapy, in accordance with usual clinical practice at these centers. Data were collected retrospectively from patients' individual charts by participating physicians. The primary effectiveness and safety endpoints were the percentage of seizure-free patients 1 year after initiation of BRV monotherapy and the proportion of patients reporting adverse events (AEs) over the complete follow-up period. Retention rates and subpopulation analysis (levetiracetam switchers, elderly and different etiologies) were also investigated.
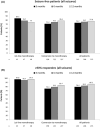
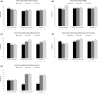
Results: A total of 276 patients were included (48 with BRV as first-line monotherapy and 228 who converted to BRV monotherapy). The overall retention rate in monotherapy at 12 months was 89.9% (87.5% for first-line monotherapy group; 90.4% for conversion-to-monotherapy group). Seizure-freedom rates at 12 months were 77.8% (75% for first-line monotherapy group; 78.4% for conversion-to-monotherapy group). AEs occurred in 39.5% of patients at 12 months (35.4% for first-line monotherapy group; 40.4% for conversion-to-monotherapy group). Most AEs were mild-to-moderate. The most frequent AEs were irritability (12.3%) and dizziness (10.1%). The most frequent AEs leading to BRV withdrawal were dizziness (1.8%) and memory problems (1.4%). Similar outcomes in terms of effectiveness and tolerability of BRV monotherapy were observed in patients switching from levetiracetam, those with different epilepsy etiologies, and elderly patients.
Significance: BRV was effective and well tolerated both as first-line monotherapy and following conversion to monotherapy in a real-world setting of patients with epilepsy.
Plain language summary: The goal of the medical treatment of epilepsy is to ensure best possible patient quality of life, by maximizing seizure control and minimizing medication toxicity. Brivaracetam (BRV) is a new-generation epilepsy treatment that is well tolerated by patients. In our study, monotherapy with BRV reduced seizures in patients who had not received other treatments and in patients who switched from a previous treatment to BRV monotherapy. BRV was well tolerated and also effective in sensitive patients (i.e., the elderly and those who had epilepsy caused by a brain tumor or a brain injury).
Keywords: anti‐seizure medication; epilepsy; monotherapy.
© 2024 The Author(s). Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
VV has received honoraria and/or research funds from Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals, Neuraxpharm, Novartis, Nutricia, Takeda, UCB Pharma and Xenon. JZ has received consultant and/or speaker honoraria from Alter, Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals, Neuraxpharm and UCB Pharma. FJLG has received consultant and/or speaker honoraria from Angelini Pharma, Bial, Eisai, Esteve, GW Pharmaceutical Company (now a part of Jazz Pharmaceuticals), LivaNova, Nutricia and UCB Pharma. XRO has received consultant and/or speaker honoraria from Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals, Livanova, Neuraxpharm and UCB Pharma. BPC has received consultant and/or speaker honoraria from Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals and UCB Pharma. BMA has received consultant and/or speaker honoraria from Angelini‐Pharma, Bial, Eisai, Kern Pharma, Jazz Pharmaceuticals, Nutricia and UCB Pharma. AGI has received consultant and/or speaker honoraria from Angelini Pharma, Bial, Eisai, Idorsia, Jazz Pharmaceuticals, Neuraxpharm and UCB Pharma. JMP has received speaker honoraria from Eisai and UCB Pharma. PMA has received honoraria and/or research funds from Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals, Kern Pharma, Livanova, Neuraxpharm and UCB Pharma. RC has received consultant and/or speaker honoraria from Angelini Pharma, Eisai, Neuraxpharm and UCB Pharma. ASM has received consultant and/or speaker honoraria from Angelini‐Pharma, Eisai, Ferrer, GW Pharmaceutical Company (now a part of Jazz Pharmaceuticals), Kern Pharma, Neuraxpharm, Nutricia, Takeda and UCB Pharma. JDH has received consultant and/or speaker honoraria from Angelini Pharma, Bial, Eisai, and UCB Pharma. JRU has received honoraria and/or research funds from Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals, Neuraxpharm, Novartis, Nutricia, Takeda, UCB Pharma and Xenon. JMS has received honoraria for advisory, educational activities, and/or research funds from Angelini Pharma, BIAL, Eisai Inc., Esteve, Ferrer, Jazz Pharmaceuticals, GW Pharmaceuticals, Sanofi, UCB Pharma, and Zogenix. The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Epilepsy [Internet] . World Health Organization Factsheet. 2024. Available from: https://www.who.int/news‐room/fact‐sheets/detail/epilepsy
-
- Kanner AM, Bicchi MM. Antiseizure medications for adults with epilepsy. JAMA. 2022;327(13):1269. - PubMed
-
- Auriel E, Landov H, Blatt I, Theitler J, Gandelman‐Marton R, Chistik V, et al. Quality of life in seizure‐free patients with epilepsy on monotherapy. Epilepsy Behav. 2009;14(1):130–133. - PubMed
-
- Rohracher A, Kalss G, Kuchukhidze G, Neuray C, Leitinger M, Höfler J, et al. New anti‐seizure medication for elderly epilepsy patients ‐ a critical narrative review. Expert Opin Pharmacother. 2021;22(5):621–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

