Circulating HBsAg-specific B cells are partially rescued in chronically HBV-infected patients with functional cure
- PMID: 39470771
- PMCID: PMC11523254
- DOI: 10.1080/22221751.2024.2409350
Circulating HBsAg-specific B cells are partially rescued in chronically HBV-infected patients with functional cure
Abstract
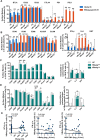
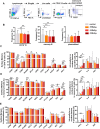
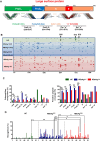
It is well established that humoral immunity targeting hepatitis B virus surface antigen (HBsAg) plays a critical role in viral clearance and clinical cure. However, the functional changes in HBsAg-specific B cells before and after achieving functional cure remain poorly understood. In this study, we characterized circulating HBsAg-specific B cells and identified functional shifts and B-cell epitopes directly associated with HBsAg loss. The phenotypes and functions of HBV-specific B cells in patients with chronic HBV infection were investigated using a dual staining method and the ELISpot assay. Epitope mapping was performed to identify B cell epitopes associated with functional cure. Hyperactivated HBsAg-specific B cells in patients who achieved HBsAg loss were composed of enriched resting memory and contracted atypical memory fractions, accompanied by sustained co-expression of multiple inhibitory receptors and increased IL-6 secretion. The frequency of HBsAb-secreting B cells was significantly increased after achieving a functional cure. The rHBsAg displayed a weaker immunomodulatory effect on B cells than rHBeAg and rHBcAg in vitro. Notably, sera from patients with HBsAg loss reacted mainly with peptides S60, S61, and S76, suggesting that these are dominant linear B-cell epitopes relevant for functional cure. Intriguingly, patients reactive with S76 showed a higher frequency of the HLA class II DQB1*05:01 allele. Taken together, HBsAg-specific B cells were partially restored in patients after achieving a functional cure. Functional cure-related epitopes may be promising targets for developing therapeutic vaccines to treat HBV infection and promote functional cure.
Keywords: B cells; B-cell epitope; Hepatitis B surface antigen; functional cure; hepatitis B surface antigen clearance.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- WHO Hepatitis B . 2024. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Accessed 2024 9 April].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
