Holmium-166 radioembolisation dosimetry in HCC
- PMID: 39470786
- PMCID: PMC11754330
- DOI: 10.1007/s00259-024-06940-2
Holmium-166 radioembolisation dosimetry in HCC
Abstract
Purpose: To evaluate dosimetry, dose-response and dose-toxicity relationships for holmium-166 (166Ho) radioembolisation in patients with hepatocellular carcinoma (HCC).
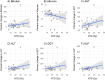
Methods: Thirty-one patients with hepatocellular carcinoma were included in the HEPAR Primary study (NCT03379844, registered on December 20th, 2017) and underwent 166Ho-microspheres radioembolisation. Linear mixed models assessed the association between tumour absorbed doses and response based on mRECIST both on tumour and patient level. Preliminary tumour absorbed dose thresholds were estimated based on predictive value. Linear regression models assessed the association between non-tumour absorbed dose and Common Terminology Criteria for Adverse Events version 4.03.
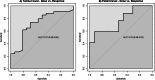
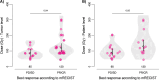
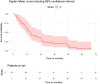
Results: Median tumour absorbed dose (tumour level) was 95.5 Gy (range 44-332 Gy). Median non-tumour absorbed dose based on whole liver volume was 19 Gy (range 3 - 48 Gy) and based on target liver volume was 30 Gy (range 13 - 54 Gy). There was a significant association between non-tumour absorbed dose and toxicity. Tumours with partial response/complete response (PR/CR, responders) received a 41% higher absorbed dose than tumours with progressive disease/stable disease (PD/SD, non-responders) (95%CI: 2%-93%, p = 0.04). A predictive value of 90% for tumour response was observed at a tumour absorbed dose threshold of 155 Gy, 100% predictive value was achieved at 184.5 Gy.
Conclusion: This study confirms a positive relationship between tumour absorbed dose and response and between non-tumour absorbed dose and toxicity. Dose thresholds found in this study can serve as a basis for personalized dosimetry in HCC patients treated with 166Ho-microspheres.
Keywords: Biodistribution; Dosimetry; Hepatocellular carcinoma; Holmium; Personalised treatment planning; Radioembolisation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This clinical study was approved by the Medical Ethics Committee of University Medical Centre Utrecht and Erasmus Medical Centre and by the institutional radiation protection committee. Furthermore, this study was performed in concordance with Good Clinical Practice and the declaration of Helsinki. Consent to participate: All participants provided written, informed consent before undergoing any study-specific activity. Conflicts of interest: Margot Reinders-Hut is currently employed by Genmab B.V., acted as a speaker for Boston Scientific. Marnix Lam acts as a consultant for Terumo, Quirem Medical and Boston Scientific, receives research support from Terumo, Quirem Medical and Boston Scientific. Maarten Smits and Arthur Braat are consultants for Terumo. The department of Radiology and Nuclear Medicine of the University Medical Centre Utrecht receives royalty payments from Quirem Medical.
Figures
References
-
- Smits MLJ, Dassen MG, Prince JF, Braat A, Beijst C, Bruijnen RCG, et al. The superior predictive value of (166)Ho-scout compared with (99m)Tc-macroaggregated albumin prior to (166)Ho-microspheres radioembolization in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2019. 10.1007/s00259-019-04460-y. - PMC - PubMed
-
- Smits MLJ, Nijsen JFW, van den Bosch MAAJ, Lam MGEH, Vente MAD, Mali WPTM, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13:1025–34. 10.1016/s1470-2045(12)70334-0. - PubMed
-
- Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. 10.1016/s2468-1253(20)30290-9. - PubMed
-
- van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat A, de Jong H, et al. Dose-Effect Relationships of (166)Ho Radioembolization in Colorectal Cancer. J Nucl Med. 2021;62:272–9. 10.2967/jnumed.120.243832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

