Hematopoietic Stem Cell Transplantation for C1q Deficiency: A Study on Behalf of the EBMT Inborn Errors Working Party
- PMID: 39470951
- PMCID: PMC11522153
- DOI: 10.1007/s10875-024-01819-1
Hematopoietic Stem Cell Transplantation for C1q Deficiency: A Study on Behalf of the EBMT Inborn Errors Working Party
Abstract
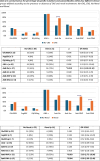
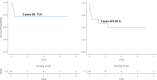
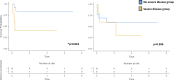
C1q deficiency is a rare inborn error of immunity characterized by increased susceptibility to infections and autoimmune manifestations mimicking SLE, with an associated morbidity and mortality. Because C1q is synthesized by monocytes, to date, four patients treated with allogeneic HSCT have been reported, with a positive outcome in three. We conducted an international retrospective study to assess the outcome of HSCT in C1q deficiency. Eighteen patients, fourteen previously unreported, from eleven referral centres, were included. Two patients had two HSCTs, thus 20 HSCTs were performed in total, at a median age of 10 years (range 0.9-19). Indications for HSCT were autoimmune manifestations not controlled by ongoing treatment in seventeen, and early development of MALT lymphoma in one patient. Overall survival (OS) was 71% and event-free survival was 59% at two years (considering an event as acute GvHD ≥ grade III, disease recurrence and death). In eleven patients HSCT led to resolution of autoimmune features and discontinuation of immunosuppressive treatments (follow-up time range 3-84 months). Five patients died due to transplant-related complications. Patients with a severe autoimmune phenotype, defined as neurological and/or renal involvement, had the worst OS (40% vs 84%; p = 0.034). Reviewing data of 69 genetically confirmed C1q deficient patients, we found that anti-Ro antibodies are associated with neurologic involvement, and anti-RNP and anti-DNA antibodies with renal involvement. In conclusion, HSCT may be a valid curative option for C1q deficiency, but careful selection of patients, with an accurate assessment of risk and benefit, is mandatory.
Keywords: Allogeneic HSCT; C1q deficiency; SLE.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

