Highly neurogenic glia from human and mouse myenteric ganglia generate functional neurons following culture and transplantation into the gut
- PMID: 39471175
- PMCID: PMC11697211
- DOI: 10.1016/j.celrep.2024.114919
Highly neurogenic glia from human and mouse myenteric ganglia generate functional neurons following culture and transplantation into the gut
Abstract
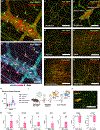
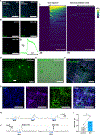
Enteric neural stem cell (ENSC) therapy offers great promise for neurointestinal diseases; however, current isolation methods yield insufficient neurons for regenerative applications. Multiomic profiling of enteric glial cells (EGCs) suggests that subpopulations within myenteric ganglia (MyGa) are a reservoir of highly neurogenic ENSCs. Here, we describe protocols to enrich for intraganglionic EGCs by isolating intact fragments of MyGa, generating cultures with higher neuronal purity than traditional methodologies isolating intramuscular single cells (IM-SCs). MyGa-derived EGCs transdifferentiate into more neurons than IM-SC-derived EGCs do, confirming their neurogenic predisposition. Following transplantation to the mouse intestine, MyGa-derived neurons generate calcium transients and activate smooth muscle in response to optogenetic stimulation. In the human intestine, MyGa-derived cells are similarly highly neurogenic, are enriched for a distinct progenitor population identified by single-cell RNA sequencing (scRNA-seq), and exhibit neuromuscular connectivity following xenogeneic transplantation into mice. Highly neurogenic ENSCs are preferentially located within the MyGa, and their selective isolation offers considerable potential for therapy.
Keywords: CP: Neuroscience; CP: Stem cell research; cell therapy; enteric glial cell; enteric nervous system; enteric neural stem cell; enteric neuron; myenteric ganglia; neurogenesis; neuroregeneration; postnatal; regeneration.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.S., A.M.G., and R.H. are inventors on US provisional patent application 63/659,137 submitted by The General Hospital Corporation that covers “Isolation of enteric neurons and progenitors from the enteric ganglia for cell therapy.”
Figures





References
-
- Furness JB (2012). The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol 9, 286–294. - PubMed
-
- Furness JB (2006). The Enteric Nervous System (Blackwell Publishing; ).
-
- Goldstein AM, Thapar N, Karunaratne TB, and De Giorgio R (2016). Clinical aspects of neurointestinal disease: pathophysiology, diagnosis, and treatment. Dev. Biol 417, 217–228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

