The future of transcranial ultrasound as a precision brain interface
- PMID: 39471185
- PMCID: PMC11521279
- DOI: 10.1371/journal.pbio.3002884
The future of transcranial ultrasound as a precision brain interface
Abstract
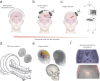
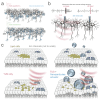
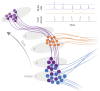
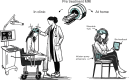
Our understanding of brain circuit operations and disorders has rapidly outpaced our ability to intervene and restore them. Developing technologies that can precisely interface with any brain region and circuit may combine diagnostics with therapeutic intervention, expediting personalised brain medicine. Transcranial ultrasound stimulation (TUS) is a promising noninvasive solution to this challenge, offering focal precision and scalability. By exploiting the biomechanics of pressure waves on brain tissue, TUS enables multi-site targeted neuromodulation across distributed circuits in the cortex and deeper areas alike. In this Essay, we explore the emergent evidence that TUS can functionally test and modify dysfunctional regions, effectively serving as a search and rescue tool for the brain. We define the challenges and opportunities faced by TUS as it moves towards greater target precision and integration with advanced brain monitoring and interventional technology. Finally, we propose a roadmap for the evolution of TUS as it progresses from a research tool to a clinically validated therapeutic for brain disorders.
Copyright: © 2024 Murphy, Fouragnan. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
We have read the journal’s policy and the author K.M. of this manuscript has the following competing interests: K.M. is a co-founder and shareholder of Attune Neuroscience.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources

