BiP/GRP78 is a pro-viral factor for diverse dsDNA viruses that promotes the survival and proliferation of cells upon KSHV infection
- PMID: 39471213
- PMCID: PMC11548844
- DOI: 10.1371/journal.ppat.1012660
BiP/GRP78 is a pro-viral factor for diverse dsDNA viruses that promotes the survival and proliferation of cells upon KSHV infection
Abstract
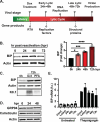
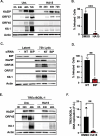
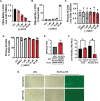
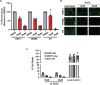
The Endoplasmic Reticulum (ER)-resident HSP70 chaperone BiP (HSPA5) plays a crucial role in maintaining and restoring protein folding homeostasis in the ER. BiP's function is often dysregulated in cancer and virus-infected cells, conferring pro-oncogenic and pro-viral advantages. We explored BiP's functions during infection by the Kaposi's sarcoma-associated herpesvirus (KSHV), an oncogenic gamma-herpesvirus associated with cancers of immunocompromised patients. Our findings reveal that BiP protein levels are upregulated in infected epithelial cells during the lytic phase of KSHV infection. This upregulation occurs independently of the unfolded protein response (UPR), a major signaling pathway that regulates BiP availability. Genetic and pharmacological inhibition of BiP halts KSHV viral replication and reduces the proliferation and survival of KSHV-infected cells. Notably, inhibition of BiP limits the spread of other alpha- and beta-herpesviruses and poxviruses with minimal toxicity for normal cells. Our work suggests that BiP is a potential target for developing broad-spectrum antiviral therapies against double-stranded DNA viruses and a promising candidate for therapeutic intervention in KSHV-related malignancies.
Copyright: © 2024 Najarro et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

