A molecular view of peptoid-induced acceleration of calcite growth
- PMID: 39471223
- PMCID: PMC11551417
- DOI: 10.1073/pnas.2412358121
A molecular view of peptoid-induced acceleration of calcite growth
Abstract
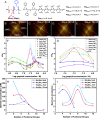
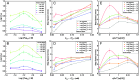
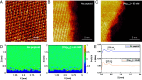
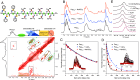
The extensive deposits of calcium carbonate (CaCO3) generated by marine organisms constitute the largest and oldest carbon dioxide (CO2) reservoir. These organisms utilize macromolecules like peptides and proteins to facilitate the nucleation and growth of carbonate minerals, serving as an effective method for CO2 sequestration. However, the precise mechanisms behind this process remain elusive. In this study, we report the use of sequence-defined peptoids, a class of peptidomimetics, to achieve the accelerated calcite step growth kinetics with the molecular level mechanistic understanding. By designing peptoids with hydrophilic and hydrophobic blocks, we systematically investigated the acceleration in step growth rate of calcite crystals using in situ atomic force microscopy (AFM), varying peptoid sequences and concentrations, CaCO3 supersaturations, and the ratio of Ca2+/ HCO3-. Mechanistic studies using NMR, three-dimensional fast force mapping (3D FFM), and isothermal titration calorimetry (ITC) were conducted to reveal the interactions of peptoids with Ca2+ and HCO3- ions in solution, as well as the effect of peptoids on solvation and energetics of calcite crystal surface. Our results indicate the multiple roles of peptoid in facilitating HCO3- deprotonation, Ca2+ desolvation, and the disruption of interfacial hydration layers of the calcite surface, which collectively contribute to a peptoid-induced acceleration of calcite growth. These findings provide guidelines for future design of sequence-specific biomimetic polymers as crystallization promoters, offering potential applications in environmental remediation (such as CO2 sequestration), biomedical engineering, and energy storage where fast crystallization is preferred.
Keywords: CO2 sequestration; atomic force microscopy; biomimetic polymer; crystal growth.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Meldrum F. C., Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 48, 187–224 (2003).
-
- Porter S. M., Seawater chemistry and early carbonate biomineralization. Science 316, 1302–1302 (2007). - PubMed
-
- Stanley S. M., Effects of global seawater chemistry on biomineralization: Past, present, and future. Chem. Rev. 108, 4483–4498 (2008). - PubMed
-
- Meldrum F. C., Coelfen H., Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 108, 4332–4432 (2008). - PubMed
-
- Sommerdijk N., de With G., Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem. Rev. 108, 4499–4550 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

