A novel N 4, N 4-dimethylcytidine in the archaeal ribosome enhances hyperthermophily
- PMID: 39471227
- PMCID: PMC11551388
- DOI: 10.1073/pnas.2405999121
A novel N 4, N 4-dimethylcytidine in the archaeal ribosome enhances hyperthermophily
Abstract
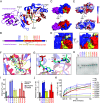
Ribosome structure and activity are challenged at high temperatures, often demanding modifications to ribosomal RNAs (rRNAs) to retain translation fidelity. LC-MS/MS, bisulfite-sequencing, and high-resolution cryo-EM structures of the archaeal ribosome identified an RNA modification, N4,N4-dimethylcytidine (m42C), at the universally conserved C918 in the 16S rRNA helix 31 loop. Here, we characterize and structurally resolve a class of RNA methyltransferase that generates m42C whose function is critical for hyperthermophilic growth. m42C is synthesized by the activity of a unique family of RNA methyltransferase containing a Rossman-fold that targets only intact ribosomes. The phylogenetic distribution of the newly identified m42C synthase family implies that m42C is biologically relevant in each domain. Resistance of m42C to bisulfite-driven deamination suggests that efforts to capture m5C profiles via bisulfite sequencing are also capturing m42C.
Keywords: N4,N4-dimethylcytidine; RNA modifications; archaea; epitranscriptome; hyperthermophile.
Conflict of interest statement
Competing interests statement:K.A.F., P.S.H., V.T., L.E., J.S., H.P.F., R.C., and T.J.S. do not have any competing financial interests nor conflicts of interest to report. N.D., E.J.W., R.T.F., Y.-L.T., G.B.R., and I.R.C. are employed and funded by New England Biolabs, Inc., a manufacturer and vendor of molecular biology reagents, including nucleic acid modifying and synthesis enzymes. The authors state that this affiliation does not affect their impartiality, objectivity of data generation or interpretation, adherence to journal standards and policies, or availability of data.
Figures



References
-
- Shigi N., et al. , Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J. Biol. Chem. 281, 2104–2113 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

