Hematopoietic cell transplant compared with standard care in adolescents and young adults with sickle cell disease
- PMID: 39471440
- PMCID: PMC11907447
- DOI: 10.1182/bloodadvances.2024013926
Hematopoietic cell transplant compared with standard care in adolescents and young adults with sickle cell disease
Abstract
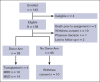
Disease-modifying therapies are standard of care (SOC) for sickle cell disease (SCD), but hematopoietic cell transplantation (HCT) has curative potential. We compared outcomes prospectively through 2 years after biologic assignment to a donor or no donor (SOC) arm based on the availability of an HLA-matched sibling or unrelated donor (BMT CTN 1503). A donor search was commenced after eligibility confirmation. The primary end point was a comparison of survival between the treatment arms 2 years after biologic assignment. Power calculations required 60 participants in the donor arm and 140 in the no donor arm to determine if early transplant-related mortality might be balanced by disease-related mortality over a longer period of follow-up. Secondary objectives were a comparison of the changes in SCD-related events, functional outcomes, and organ function. The data were analyzed according to the intent-to-treat principle. A total of 113 participants were enrolled with 28 in the donor arm and 85 in the no donor arm. The 2-year probabilities of survival were 89% and 93%, in the donor vs no donor arms. Vaso-occlusive pain (VOC) was less frequent in the donor arm in the second year after biologic assignment (P < .001). Based on PROMIS-57 surveys, there was a decrease in fatigue (P = .003) and an increase in the ability to participate in social roles and activities (P = .003) in the donor arm 2 years after biologic assignment. Differences in other secondary outcomes did not reach statistical significance. Barriers to accrual prevented an objective comparison of survival. Assignment to the donor arm led to improvements in VOC, fatigue, and social function. This trial was registered at www.clinicaltrials.gov as #NCT02766465.
© 2025 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.C.W. reports serving as a consultant for Vertex Pharmaceuticals Inc, Encoma Inc, Sanofi Inc, and BioChip Labs Inc. P.L. reports serving as a consultant for Janssen Therapeutics and receiving research funding from Bristol Myers Squibb and Marker Therapeutics. J.J.S. reports serving as a consultant for Editas Medicine. The remaining authors declare no competing financial interests.
Figures



References
-
- Pecker LH, Lanzkron S. Sickle cell disease. Ann Intern Med. 2021;174(1):Itc1–Itc16. - PubMed
-
- Chaturvedi S, Ghafuri DL, Jordan N, Kassim A, Rodeghier M, DeBaun MR. Clustering of end-organ disease and earlier mortality in adults with sickle cell disease: a retrospective-prospective cohort study. Am J Hematol. 2018;93(9):1153–1160. - PubMed

